Small GTP-binding protein Ran is regulated by posttranslational lysine acetylation
- PMID: 26124124
- PMCID: PMC4507232
- DOI: 10.1073/pnas.1505995112
Small GTP-binding protein Ran is regulated by posttranslational lysine acetylation
Abstract
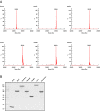
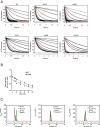
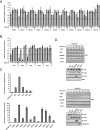
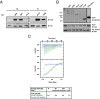
Ran is a small GTP-binding protein of the Ras superfamily regulating fundamental cellular processes: nucleo-cytoplasmic transport, nuclear envelope formation and mitotic spindle assembly. An intracellular Ran•GTP/Ran•GDP gradient created by the distinct subcellular localization of its regulators RCC1 and RanGAP mediates many of its cellular effects. Recent proteomic screens identified five Ran lysine acetylation sites in human and eleven sites in mouse/rat tissues. Some of these sites are located in functionally highly important regions such as switch I and switch II. Here, we show that lysine acetylation interferes with essential aspects of Ran function: nucleotide exchange and hydrolysis, subcellular Ran localization, GTP hydrolysis, and the interaction with import and export receptors. Deacetylation activity of certain sirtuins was detected for two Ran acetylation sites in vitro. Moreover, Ran was acetylated by CBP/p300 and Tip60 in vitro and on transferase overexpression in vivo. Overall, this study addresses many important challenges of the acetylome field, which will be discussed.
Keywords: Ran; genetic code expansion concept; lysine acetylation; nuclear cytosolic transport; nucleus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Clarke PR, Zhang C. Spatial and temporal coordination of mitosis by Ran GTPase. Nat Rev Mol Cell Biol. 2008;9(6):464–477. - PubMed
-
- Scheffzek K, Klebe C, Fritz-Wolf K, Kabsch W, Wittinghofer A. Crystal structure of the nuclear Ras-related protein Ran in its GDP-bound form. Nature. 1995;374(6520):378–381. - PubMed
-
- Monecke T, et al. Crystal structure of the nuclear export receptor CRM1 in complex with Snurportin1 and RanGTP. Science. 2009;324(5930):1087–1091. - PubMed
-
- Stewart M, Kent HM, McCoy AJ. Structural basis for molecular recognition between nuclear transport factor 2 (NTF2) and the GDP-bound form of the Ras-family GTPase Ran. J Mol Biol. 1998;277(3):635–646. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

