Structural asymmetry in a conserved signaling system that regulates division, replication, and virulence of an intracellular pathogen
- PMID: 26124143
- PMCID: PMC4507200
- DOI: 10.1073/pnas.1503118112
Structural asymmetry in a conserved signaling system that regulates division, replication, and virulence of an intracellular pathogen
Abstract
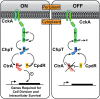
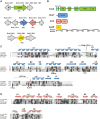
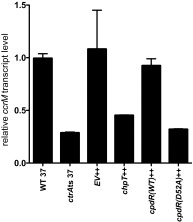
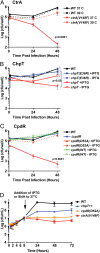
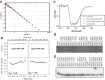
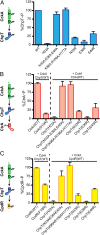
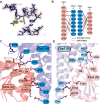
We have functionally and structurally defined an essential protein phosphorelay that regulates expression of genes required for growth, division, and intracellular survival of the global zoonotic pathogen Brucella abortus. Our study delineates phosphoryl transfer through this molecular pathway, which initiates from the sensor kinase CckA and proceeds through the ChpT phosphotransferase to two regulatory substrates: CtrA and CpdR. Genetic perturbation of this system results in defects in cell growth and division site selection, and a specific viability deficit inside human phagocytic cells. Thus, proper control of B. abortus division site polarity is necessary for survival in the intracellular niche. We further define the structural foundations of signaling from the central phosphotransferase, ChpT, to its response regulator substrate, CtrA, and provide evidence that there are at least two modes of interaction between ChpT and CtrA, only one of which is competent to catalyze phosphoryltransfer. The structure and dynamics of the active site on each side of the ChpT homodimer are distinct, supporting a model in which quaternary structure of the 2:2 ChpT-CtrA complex enforces an asymmetric mechanism of phosphoryl transfer between ChpT and CtrA. Our study provides mechanistic understanding, from the cellular to the atomic scale, of a conserved transcriptional regulatory system that controls the cellular and infection biology of B. abortus. More generally, our results provide insight into the structural basis of two-component signal transduction, which is broadly conserved in bacteria, plants, and fungi.
Keywords: Brucella abortus; ChpT; CtrA; cell cycle; two-component system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6(2):91–99. - PubMed
-
- Atluri VL, Xavier MN, de Jong MF, den Hartigh AB, Tsolis RM. Interactions of the human pathogenic Brucella species with their hosts. Annu Rev Microbiol. 2011;65:523–541. - PubMed
-
- Batut J, Andersson SG, O’Callaghan D. The evolution of chronic infection strategies in the alpha-proteobacteria. Nat Rev Microbiol. 2004;2(12):933–945. - PubMed
-
- Philippot L, et al. The ecological coherence of high bacterial taxonomic ranks. Nat Rev Microbiol. 2010;8(7):523–529. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

