Enzyme mechanism-based, oxidative DNA-protein cross-links formed with DNA polymerase β in vivo
- PMID: 26124145
- PMCID: PMC4507217
- DOI: 10.1073/pnas.1501101112
Enzyme mechanism-based, oxidative DNA-protein cross-links formed with DNA polymerase β in vivo
Abstract
Free radical attack on the C1' position of DNA deoxyribose generates the oxidized abasic (AP) site 2-deoxyribonolactone (dL). Upon encountering dL, AP lyase enzymes such as DNA polymerase β (Polβ) form dead-end, covalent intermediates in vitro during attempted DNA repair. However, the conditions that lead to the in vivo formation of such DNA-protein cross-links (DPC), and their impact on cellular functions, have remained unknown. We adapted an immuno-slot blot approach to detect oxidative Polβ-DPC in vivo. Treatment of mammalian cells with genotoxic oxidants that generate dL in DNA led to the formation of Polβ-DPC in vivo. In a dose-dependent fashion, Polβ-DPC were detected in MDA-MB-231 human cells treated with the antitumor drug tirapazamine (TPZ; much more Polβ-DPC under 1% O2 than under 21% O2) and even more robustly with the "chemical nuclease" 1,10-copper-ortho-phenanthroline, Cu(OP)2. Mouse embryonic fibroblasts challenged with TPZ or Cu(OP)2 also incurred Polβ-DPC. Nonoxidative agents did not generate Polβ-DPC. The cross-linking in vivo was clearly a result of the base excision DNA repair pathway: oxidative Polβ-DPC depended on the Ape1 AP endonuclease, which generates the Polβ lyase substrate, and they required the essential lysine-72 in the Polβ lyase active site. Oxidative Polβ-DPC had an unexpectedly short half-life (∼ 30 min) in both human and mouse cells, and their removal was dependent on the proteasome. Proteasome inhibition under Cu(OP)2 treatment was significantly more cytotoxic to cells expressing wild-type Polβ than to cells with the lyase-defective form. That observation underscores the genotoxic potential of oxidative Polβ-DPC and the biological pressure to repair them.
Keywords: 2-deoxyribonolactone; AP lyase; abasic site; base excision repair; free radical damage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
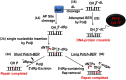


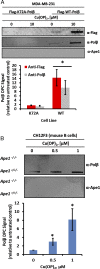
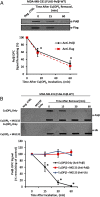

Similar articles
-
Oxidative DNA-protein crosslinks formed in mammalian cells by abasic site lyases involved in DNA repair.DNA Repair (Amst). 2020 Mar;87:102773. doi: 10.1016/j.dnarep.2019.102773. Epub 2020 Jan 9. DNA Repair (Amst). 2020. PMID: 31945542 Free PMC article.
-
When DNA repair goes wrong: BER-generated DNA-protein crosslinks to oxidative lesions.DNA Repair (Amst). 2016 Aug;44:103-109. doi: 10.1016/j.dnarep.2016.05.014. Epub 2016 May 20. DNA Repair (Amst). 2016. PMID: 27264558 Free PMC article. Review.
-
C-terminal residues of DNA polymerase β and E3 ligase required for ubiquitin-linked proteolysis of oxidative DNA-protein crosslinks.DNA Repair (Amst). 2024 Nov;143:103756. doi: 10.1016/j.dnarep.2024.103756. Epub 2024 Aug 28. DNA Repair (Amst). 2024. PMID: 39243487
-
Long-patch base excision DNA repair of 2-deoxyribonolactone prevents the formation of DNA-protein cross-links with DNA polymerase beta.J Biol Chem. 2005 Nov 25;280(47):39095-103. doi: 10.1074/jbc.M506480200. Epub 2005 Sep 27. J Biol Chem. 2005. PMID: 16188889
-
Roles of base excision repair subpathways in correcting oxidized abasic sites in DNA.FEBS J. 2006 Apr;273(8):1620-9. doi: 10.1111/j.1742-4658.2006.05192.x. FEBS J. 2006. PMID: 16623699 Review.
Cited by
-
Excessive Reactive Oxygen Species and Exotic DNA Lesions as an Exploitable Liability.Biochemistry. 2016 Sep 27;55(38):5341-52. doi: 10.1021/acs.biochem.6b00703. Epub 2016 Sep 13. Biochemistry. 2016. PMID: 27582430 Free PMC article. Review.
-
Formation and repair of DNA-protein crosslink damage.Sci China Life Sci. 2017 Oct;60(10):1065-1076. doi: 10.1007/s11427-017-9183-4. Epub 2017 Oct 30. Sci China Life Sci. 2017. PMID: 29098631 Free PMC article. Review.
-
Oxidative DNA-protein crosslinks formed in mammalian cells by abasic site lyases involved in DNA repair.DNA Repair (Amst). 2020 Mar;87:102773. doi: 10.1016/j.dnarep.2019.102773. Epub 2020 Jan 9. DNA Repair (Amst). 2020. PMID: 31945542 Free PMC article.
-
DNA Structure-Specific Cleavage of DNA-Protein Crosslinks by the SPRTN Protease.Mol Cell. 2020 Oct 1;80(1):102-113.e6. doi: 10.1016/j.molcel.2020.08.003. Epub 2020 Aug 26. Mol Cell. 2020. PMID: 32853547 Free PMC article.
-
Mitochondrial DNA degradation: A quality control measure for mitochondrial genome maintenance and stress response.Enzymes. 2019;45:311-341. doi: 10.1016/bs.enz.2019.08.004. Enzymes. 2019. PMID: 31627882 Free PMC article. Review.
References
-
- Rhaese H-J, Freese E. Chemical analysis of DNA alterations. I. Base liberation and backbone breakage of DNA and oligodeoxyadenylic acid induced by hydrogen peroxide and hydroxylamine. Biochim Biophys Acta. 1968;155(2):476–490. - PubMed
-
- Meijler MM, Zelenko O, Sigman DS. Chemical mechanism of DNA scission by (1,10-phenanthroline)copper. Carbonyl oxygen of 5-methylenefuranone is derived from water. J Am Chem Soc. 1997;119(5):1135–1136.
-
- Pitié M, et al. DNA cleavage by copper complexes of 2- and 3-Clip-Phen derivatives. Eur J Inorg Chem. 2003;2003(3):528–540.
-
- Kappen LS, Goldberg IH. Identification of 2-deoxyribonolactone at the site of neocarzinostatin-induced cytosine release in the sequence d(AGC) Biochemistry. 1989;28(3):1027–1032. - PubMed
-
- Daniels JS, Gates KS, Tronche C, Greenberg MM. Direct evidence for bimodal DNA damage induced by tirapazamine. Chem Res Toxicol. 1998;11(11):1254–1257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

