Active and widespread halogen chemistry in the tropical and subtropical free troposphere
- PMID: 26124148
- PMCID: PMC4522746
- DOI: 10.1073/pnas.1505142112
Active and widespread halogen chemistry in the tropical and subtropical free troposphere
Abstract
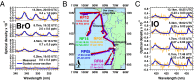
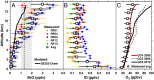
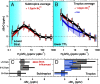
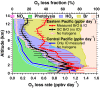
Halogens in the troposphere are increasingly recognized as playing an important role for atmospheric chemistry, and possibly climate. Bromine and iodine react catalytically to destroy ozone (O3), oxidize mercury, and modify oxidative capacity that is relevant for the lifetime of greenhouse gases. Most of the tropospheric O3 and methane (CH4) loss occurs at tropical latitudes. Here we report simultaneous measurements of vertical profiles of bromine oxide (BrO) and iodine oxide (IO) in the tropical and subtropical free troposphere (10 °N to 40 °S), and show that these halogens are responsible for 34% of the column-integrated loss of tropospheric O3. The observed BrO concentrations increase strongly with altitude (∼ 3.4 pptv at 13.5 km), and are 2-4 times higher than predicted in the tropical free troposphere. BrO resembles model predictions more closely in stratospheric air. The largest model low bias is observed in the lower tropical transition layer (TTL) over the tropical eastern Pacific Ocean, and may reflect a missing inorganic bromine source supplying an additional 2.5-6.4 pptv total inorganic bromine (Bry), or model overestimated Bry wet scavenging. Our results highlight the importance of heterogeneous chemistry on ice clouds, and imply an additional Bry source from the debromination of sea salt residue in the lower TTL. The observed levels of bromine oxidize mercury up to 3.5 times faster than models predict, possibly increasing mercury deposition to the ocean. The halogen-catalyzed loss of tropospheric O3 needs to be considered when estimating past and future ozone radiative effects.
Keywords: UTLS; atmospheric chemistry; halogens; heterogeneous chemistry; oxidative capacity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Read KA, et al. Extensive halogen-mediated ozone destruction over the tropical Atlantic Ocean. Nature. 2008;453(7199):1232–1235. - PubMed
-
- von Glasow R, von Kuhlmann R, Lawrence M, Platt U, Crutzen P. Impact of reactive bromine chemistry in the troposphere. Atmos Chem Phys. 2004;4(11/12):2481–2497.
-
- Saiz-Lopez A, et al. Estimating the climate significance of halogen-driven ozone loss in the tropical marine troposphere. Atmos Chem Phys. 2012;12(9):3939–3949.
-
- Holmes CD, Jacob DJ, Mason RP, Jaffe DA. Sources and deposition of reactive gaseous mercury in the marine atmosphere. Atmos Environ. 2009;43(14):2278–2285.
-
- Hynes A, Donohoue D, Goodsite M, Hedgecock I. Our current understanding of major chemical and physical processes affecting mercury dynamics in the atmosphere and at the air-water/terrestrial interfaces. In: Mason R, Pirrone N, editors. Mercury Fate and Transport in the Global Atmosphere. Springer; New York: 2009. pp. 427–457.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

