Phase I Imaging and Pharmacodynamic Trial of CS-1008 in Patients With Metastatic Colorectal Cancer
- PMID: 26124477
- PMCID: PMC4881374
- DOI: 10.1200/JCO.2014.60.4256
Phase I Imaging and Pharmacodynamic Trial of CS-1008 in Patients With Metastatic Colorectal Cancer
Abstract
Purpose: CS-1008 (tigatuzumab) is a humanized, monoclonal immunoglobulin G1 (IgG1) agonistic antibody to human death receptor 5. The purpose of this study was to investigate the impact of CS-1008 dose on the biodistribution, quantitative tumor uptake, and antitumor response in patients with metastatic colorectal cancer (mCRC).
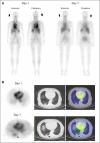
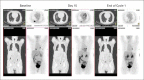
Patients and methods: Patients with mCRC who had received at least one course of chemotherapy were assigned to one of five dosage cohorts and infused with a weekly dose of CS-1008. Day 1 and day 36 doses were trace-labeled with indium-111 ((111)In), followed by whole-body planar and regional single-photon emission computed tomography (SPECT) imaging at several time points over the course of 10 days.
Results: Nineteen patients were enrolled. (111)In-CS-1008 uptake in tumor was observed in only 12 patients (63%). (111)In-CS-1008 uptake and pharmacokinetics were not affected by dose or repeated drug administration. (111)In-CS-1008 biodistribution showed gradual blood-pool clearance and no abnormal uptake in normal tissue. No anti-CS-1008 antibody development was detected. One patient achieved partial response (3.7 months duration), eight patients had stable disease, and 10 patients had progressive disease. Clinical benefit rate (stable disease + partial response) in patients with (111)In-CS-1008 uptake in tumor was 58% versus 28% in patients with no uptake. An analysis of individual lesions showed that lesions with antibody uptake were one third as likely to progress as those without antibody uptake (P = .07). Death-receptor-5 expression in archived tumor samples did not correlate with (111)In-CS-1008 uptake (P = .5) or tumor response (P = .6).
Conclusion: Death-receptor-5 imaging with (111)In-CS-1008 reveals interpatient and intrapatient heterogeneity of uptake in tumor, is not dose dependent, and is predictive of clinical benefit in the treatment of patients who have mCRC.
Trial registration: ClinicalTrials.gov NCT01220999.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures

Comment in
-
Molecular Imaging As a Tool for Drug Development and Trial Design.J Clin Oncol. 2015 Aug 20;33(24):2585-7. doi: 10.1200/JCO.2015.61.6425. Epub 2015 Jul 13. J Clin Oncol. 2015. PMID: 26169612 No abstract available.
References
-
- de Vries EGE, Gietema JA, de Jong S. Tumor necrosis factor-related apoptosis-inducing ligand pathway and its therapeutic implications. Clin Cancer Res. 2006;12:2390–2393. - PubMed
-
- Daniels RA, Turley H, Kimberley FC, et al. Expression of TRAIL and TRAIL receptors in normal and malignant tissues. Cell Res. 2005;15:430–438. - PubMed
-
- Ichikawa K, Liu W, Zhao L, et al. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat Med. 2001;7:954–960. - PubMed
-
- Yada A, Yazawa M, Ishida S, et al. A novel humanized anti-human death receptor 5 antibody CS-1008 induces apoptosis in tumor cells without toxicity in hepatocytes. Ann Oncol. 2008;19:1060–1067. - PubMed
-
- Buchsbaum DJ, Zhou T, Grizzle WE, et al. Antitumor efficacy of TRA-8 anti-DR5 monoclonal antibody alone or in combination with chemotherapy and/or radiation therapy in a human breast cancer model. Clin Cancer Res. 2003;9:3731–3741. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

