Early Relapse of Follicular Lymphoma After Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Defines Patients at High Risk for Death: An Analysis From the National LymphoCare Study
- PMID: 26124482
- PMCID: PMC4879714
- DOI: 10.1200/JCO.2014.59.7534
Early Relapse of Follicular Lymphoma After Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Defines Patients at High Risk for Death: An Analysis From the National LymphoCare Study
Erratum in
- J Clin Oncol. 2016 Apr 20;34(12):1430
-
ERRATUM.J Clin Oncol. 2016 Apr 20;34(12):1430. doi: 10.1200/JCO.2016.67.4879. J Clin Oncol. 2016. PMID: 31265520 Free PMC article.
Abstract
Purpose: Twenty percent of patients with follicular lymphoma (FL) experience progression of disease (POD) within 2 years of initial chemoimmunotherapy. We analyzed data from the National LymphoCare Study to identify whether prognostic FL factors are associated with early POD and whether patients with early POD are at high risk for death.
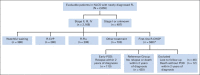
Patients and methods: In total, 588 patients with stage 2 to 4 FL received first-line rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). Two groups were defined: patients with early POD 2 years or less after diagnosis and those without POD within 2 years, the reference group. An independent validation set, 147 patients with FL who received first-line R-CHOP, was analyzed for reproducibility.
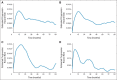
Results: Of 588 patients, 19% (n = 110) had early POD, 71% (n = 420) were in the reference group, 8% (n = 46) were lost to follow-up, and 2% (n = 12) died without POD less than 2 years after diagnosis. Five-year overall survival was lower in the early-POD group than in the reference group (50% v 90%). This trend was maintained after we adjusted for FL International Prognostic Index (hazard ratio, 6.44; 95% CI, 4.33 to 9.58). Results were similar for the validation set (FL International Prognostic Index-adjusted hazard ratio, 19.8).
Conclusion: In patients with FL who received first-line R-CHOP, POD within 2 years after diagnosis was associated with poor outcomes and should be further validated as a standard end point of chemoimmunotherapy trials of untreated FL. This high-risk FL population warrants further study in directed prospective clinical trials.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Figures

Comment in
-
Rethinking Prognosis and Therapy for Follicular Lymphoma.J Clin Oncol. 2015 Aug 10;33(23):2489-91. doi: 10.1200/JCO.2015.62.3256. Epub 2015 Jul 13. J Clin Oncol. 2015. PMID: 26169620 No abstract available.
References
-
- Zelenetz AD, Abramson JS, Advani RH, et al. Non-Hodgkin's lymphomas. J Natl Compr Canc Netw. 2011;9:484–560. - PubMed
-
- Liu Q, Fayad L, Cabanillas F, et al. Improvement of overall and failure-free survival in stage IV follicular lymphoma: 25 years of treatment experience at The University of Texas MD Anderson Cancer Center. J Clin Oncol. 2006;24:1582–1589. - PubMed
-
- Fisher RI, LeBlanc M, Press OW, et al. New treatment options have changed the survival of patients with follicular lymphoma. J Clin Oncol. 2005;23:8447–8452. - PubMed
-
- Swenson WT, Wooldridge JE, Lynch CF, et al. Improved survival of follicular lymphoma patients in the United States. J Clin Oncol. 2005;23:5019–5026. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

