Correlation of mutation profile and response in patients with myelofibrosis treated with ruxolitinib
- PMID: 26124496
- PMCID: PMC4528066
- DOI: 10.1182/blood-2015-03-633404
Correlation of mutation profile and response in patients with myelofibrosis treated with ruxolitinib
Abstract
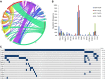
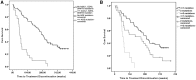
Although most patients with myelofibrosis (MF) derive benefit from ruxolitinib, some are refractory, have a suboptimal response, or quickly lose their response. To identify genes that may predict response to ruxolitinib, we performed targeted next-generation sequencing (NGS) of a panel of 28 genes recurrently mutated in hematologic malignancies in a cohort of patients with MF who were treated with ruxolitinib in a phase 1/2 study. We also tested for CALR deletions by standard polymerase chain reaction methods. Ninety-eight percent of patients had a mutation in ≥1 gene. Seventy-nine (82.1%) patients had the JAK2(V617F) mutation, 9 (9.5%) had CALR mutations (7 type 1, 2 type 2), 3 (3.1%) had MPL mutations, and 4 (4.2%) were negative for all 3. ASXL1/JAK2 and TET2/JAK2 were the most frequently comutated genes. Mutations in NRAS, KRAS, PTPN11, GATA2, TP53, and RUNX1 were found in <5% of patients. Spleen response (≥50% reduction in palpable spleen size) was inversely correlated with the number of mutations; patients with ≤2 mutations had ninefold higher odds of a spleen response than those with ≥3 mutations (odds ratio = 9.37; 95% confidence interval, 1.86-47.2). Patients with ≥3 mutations also had a shorter time to treatment discontinuation and shorter overall survival than those with fewer mutations. In multivariable analysis, only number of mutations and spleen response remained associated with time to treatment discontinuation. Patients with ≥3 mutations had the worst outcomes, suggesting that multigene profiling may be useful for therapeutic planning for MF.
© 2015 by The American Society of Hematology.
Figures
References
-
- Guglielmelli P, Biamonte F, Rotunno G, et al. COMFORT-II Investigators; Associazione Italiana per la Ricerca sul Cancro Gruppo Italiano Malattie Mieloproliferative (AGIMM) Investigators. Impact of mutational status on outcomes in myelofibrosis patients treated with ruxolitinib in the COMFORT-II study. Blood. 2014;123(14):2157–2160. - PubMed
-
- Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–798. - PubMed
-
- Cazzola M, Kralovics R. From Janus kinase 2 to calreticulin: the clinically relevant genomic landscape of myeloproliferative neoplasms. Blood. 2014;123(24):3714-3719. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

