Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry
- PMID: 26124708
- PMCID: PMC4466441
- DOI: 10.3389/fnsys.2015.00090
Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry
Abstract
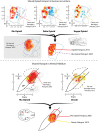
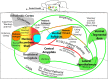
The study of the neural bases of eating behavior, hunger, and reward has consistently implicated the lateral hypothalamus (LH) and its interactions with mesocorticolimbic circuitry, such as mesolimbic dopamine projections to nucleus accumbens (NAc) and ventral pallidum (VP), in controlling motivation to eat. The NAc and VP play special roles in mediating the hedonic impact ("liking") and motivational incentive salience ("wanting") of food rewards, and their interactions with LH help permit regulatory hunger/satiety modulation of food motivation and reward. Here, we review some progress that has been made regarding this circuitry and its functions: the identification of localized anatomical hedonic hotspots within NAc and VP for enhancing hedonic impact; interactions of NAc/VP hedonic hotspots with specific LH signals such as orexin; an anterior-posterior gradient of sites in NAc shell for producing intense appetitive eating vs. intense fearful reactions; and anatomically distributed appetitive functions of dopamine and mu opioid signals in NAc shell and related structures. Such findings help improve our understanding of NAc, VP, and LH interactions in mediating affective and motivation functions, including "liking" and "wanting" for food rewards.
Keywords: lateral hypothalamus; motivation; nucleus accumbens; reward; ventral pallidum.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

