Microbial electron transport and energy conservation - the foundation for optimizing bioelectrochemical systems
- PMID: 26124754
- PMCID: PMC4463002
- DOI: 10.3389/fmicb.2015.00575
Microbial electron transport and energy conservation - the foundation for optimizing bioelectrochemical systems
Abstract
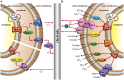
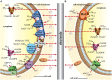
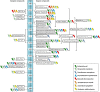
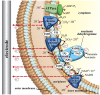
Microbial electrochemical techniques describe a variety of emerging technologies that use electrode-bacteria interactions for biotechnology applications including the production of electricity, waste and wastewater treatment, bioremediation and the production of valuable products. Central in each application is the ability of the microbial catalyst to interact with external electron acceptors and/or donors and its metabolic properties that enable the combination of electron transport and carbon metabolism. And here also lies the key challenge. A wide range of microbes has been discovered to be able to exchange electrons with solid surfaces or mediators but only a few have been studied in depth. Especially electron transfer mechanisms from cathodes towards the microbial organism are poorly understood but are essential for many applications such as microbial electrosynthesis. We analyze the different electron transport chains that nature offers for organisms such as metal respiring bacteria and acetogens, but also standard biotechnological organisms currently used in bio-production. Special focus lies on the essential connection of redox and energy metabolism, which is often ignored when studying bioelectrochemical systems. The possibility of extracellular electron exchange at different points in each organism is discussed regarding required redox potentials and effect on cellular redox and energy levels. Key compounds such as electron carriers (e.g., cytochromes, ferredoxin, quinones, flavins) are identified and analyzed regarding their possible role in electrode-microbe interactions. This work summarizes our current knowledge on electron transport processes and uses a theoretical approach to predict the impact of different modes of transfer on the energy metabolism. As such it adds an important piece of fundamental understanding of microbial electron transport possibilities to the research community and will help to optimize and advance bioelectrochemical techniques.
Keywords: ATP yield; acetogenic bacteria; bio electrochemistry; bioelectrochemical system; microbial electron transport; microbial electrosynthesis; microbial fuel cell; redox potential.
Figures
References
-
- Baltch A. L., Smith R. P. (eds) (1994). Pseudomonas aeruginosa: Infections and Treatment. New York, NY: Marcel Dekker, Inc.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

