Targeting cancer by binding iron: Dissecting cellular signaling pathways
- PMID: 26125440
- PMCID: PMC4662454
- DOI: 10.18632/oncotarget.4349
Targeting cancer by binding iron: Dissecting cellular signaling pathways
Abstract
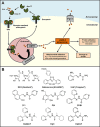
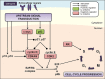
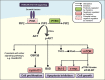
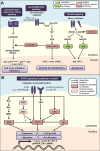
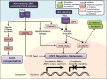
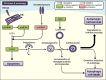
Newer and more potent therapies are urgently needed to effectively treat advanced cancers that have developed resistance and metastasized. One such strategy is to target cancer cell iron metabolism, which is altered compared to normal cells and may facilitate their rapid proliferation. This is supported by studies reporting the anti-neoplastic activities of the clinically available iron chelators, desferrioxamine and deferasirox. More recently, ligands of the di-2-pyridylketone thiosemicarbazone (DpT) class have demonstrated potent and selective anti-proliferative activity across multiple cancer-types in vivo, fueling studies aimed at dissecting their molecular mechanisms of action. In the past five years alone, significant advances have been made in understanding how chelators not only modulate cellular iron metabolism, but also multiple signaling pathways implicated in tumor progression and metastasis. Herein, we discuss recent research on the targeting of iron in cancer cells, with a focus on the novel and potent DpT ligands. Several key studies have revealed that iron chelation can target the AKT, ERK, JNK, p38, STAT3, TGF-β, Wnt and autophagic pathways to subsequently inhibit cellular proliferation, the epithelial-mesenchymal transition (EMT) and metastasis. These developments emphasize that these novel therapies could be utilized clinically to effectively target cancer.
Keywords: cancer; chelators; iron; signaling; thiosemicarbazones.
Conflict of interest statement
D.R.R. is a stakeholder in the companies, Oncochel Therapeutics LLC, USA, and Oncochel Therapeutics Pty Ltd, Australia, that are developing the thiosemicarbazone, DpC, for the treatment of cancer. D.R.R. also consults for Oncochel Therapeutics LLC and Pty Ltd.
Figures
References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Kummar S, Chen HX, Wright J, Holbeck S, Millin MD, Tomaszewski J, Zweibel J, Collins J, Doroshow JH. Utilizing targeted cancer therapeutic agents in combination: novel approaches and urgent requirements. Nat Rev Drug Discov. 2010;9:843–856. - PubMed
-
- Kalinowski DS, Richardson DR. The evolution of iron chelators for the treatment of iron overload disease and cancer. Pharmacol Rev. 2005;57:547–583. - PubMed
-
- Merlot AM, Kalinowski DS, Richardson DR. Novel chelators for cancer treatment: where are we now? Antioxid Redox Signal. 2013;18:973–1006. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

