Early diagnosis of bladder cancer through the detection of urinary tyrosine-phosphorylated proteins
- PMID: 26125446
- PMCID: PMC4522638
- DOI: 10.1038/bjc.2015.232
Early diagnosis of bladder cancer through the detection of urinary tyrosine-phosphorylated proteins
Abstract
Background: A noninvasive, highly sensitive and specific urine test is needed for bladder cancer (BC) diagnosis and surveillance in addition to the invasive cystoscopy. We previously described the diagnostic effectiveness of urinary tyrosine-phosphorylated proteins (UPY) and a new assay (UPY-A) for their measurement in a pilot study. The aim of this work was to evaluate the performances of the UPY-A using an independent cohort of 262 subjects.
Methods: Urinary tyrosine-phosphorylated proteins were measured by UPY-A test. The area under ROC curve, cutoff, sensitivity, specificity and predictive values of UPY-A were determined. The association of UPY levels with tumour staging, grading, recurrence and progression risk was analysed by Kruskal-Wallis and Wilcoxon's test. To test the probability to be a case if positive at the UPY-A, a logistic test adjusted for possible confounding factor was used.
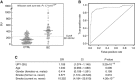
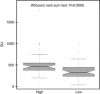
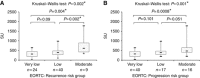
Results: Results showed a significant difference of UPY levels between patients with BC vs healthy controls. For the best cutoff value, 261.26 Standard Units (SU), the sensitivity of the assay was 80.43% and the specificity was 78.82%. A statistically significant difference was found in the levels of UPY at different BC stages and grades between Ta and T1 and with different risk of recurrence and progression. A statistically significant increased risk for BC at UPY-A ⩾261.26 SU was observed.
Conclusions: The present study supplies important information on the diagnostic characteristics of UPY-A revealing remarkable performances for early stages and allowing its potential use for different applications encompassing the screening of high-risk subjects, primary diagnosis and posttreatment surveillance.
Figures


References
-
- Al Hussain TO, Akhtar M. Molecular basis of urinary bladder cancer. Adv Anat Pathol. 2013;20:53–60. - PubMed
-
- Babjuk M, Böhle A, Burger M, Compérat E, Kaasinen E, Palou J, van Rhijn BWG, Rouprêt M, Shariat S, Sylvester R, Zigeuner R. Guidelines on non-muscle-invasive bladder cancer (Ta, T1 and CIS) Eur Assoc Urol. 2014;2014:1–48.
-
- Behrens T, Bonberg N, Casjens S, Pesch B, Brüning T. A practical guide to epidemiological practice and standards in the identification and validation of diagnostic markers using a bladder cancer example. Biochim Biophys Acta. 2014;1844:145–155. - PubMed
-
- Blume-Jensen P, Hunter T. Oncogenic kinase signaling. Nature. 2001;411:355–365. - PubMed
-
- Boman H, Hedelin H, Jacobsson S, Holmäng S. Newly diagnosed bladder cancer: the relationship of initial symptoms, degree of microhematuria and tumor marker status. J Urol. 2002;168:1955–1959. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

