Fibroblast viability and phenotypic changes within glycated stiffened three-dimensional collagen matrices
- PMID: 26126411
- PMCID: PMC4494165
- DOI: 10.1186/s12931-015-0237-z
Fibroblast viability and phenotypic changes within glycated stiffened three-dimensional collagen matrices
Erratum in
-
Erratum to: Fibroblast viability and phenotypic changes within glycated stiffened three-dimensional collagen matrices.Respir Res. 2015 Nov 2;16:136. doi: 10.1186/s12931-015-0277-4. Respir Res. 2015. PMID: 26525898 Free PMC article. No abstract available.
Abstract
Background: There is growing interest in the development of cell culture assays that enable the rigidity of the extracellular matrix to be increased. A promising approach is based on three-dimensional collagen type I matrices that are stiffened by cross-linking through non-enzymatic glycation with reducing sugars.
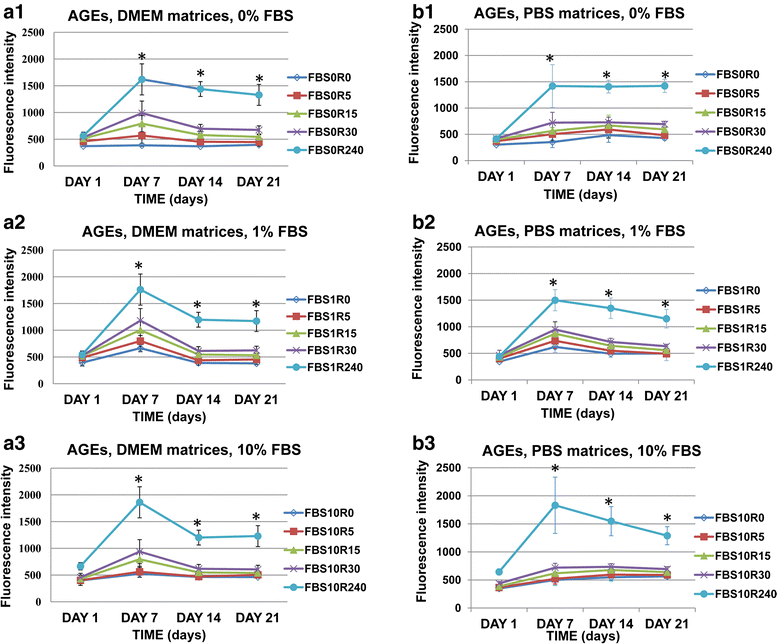
Methods: The present study evaluated the biomechanical changes in the non-enzymatically glycated type I collagen matrices, including collagen organization, the advanced glycation end products formation and stiffness achievement. Gels were glycated with ribose at different concentrations (0, 5, 15, 30 and 240 mM). The viability and the phenotypic changes of primary human lung fibroblasts cultured within the non-enzymatically glycated gels were also evaluated along three consecutive weeks. Statistical tests used for data analyze were Mann-Whitney U, Kruskal Wallis, Student's t-test, two-way ANOVA, multivariate ANOVA, linear regression test and mixed linear model.
Results: Our findings indicated that the process of collagen glycation increases the stiffness of the matrices and generates advanced glycation end products in a ribose concentration-dependent manner. Furthermore, we identified optimal ribose concentrations and media conditions for cell viability and growth within the glycated matrices. The microenvironment of this collagen based three-dimensional culture induces α-smooth muscle actin and tenascin-C fibroblast protein expression. Finally, a progressive contractile phenotype cell differentiation was associated with the contraction of these gels.
Conclusions: The use of non-enzymatic glycation with a low ribose concentration may provide a suitable model with a mechanic and oxidative modified environment with cells embedded in it, which allowed cell proliferation and induced fibroblast phenotypic changes. Such culture model could be appropriate for investigations of the behavior and phenotypic changes in cells that occur during lung fibrosis as well as for testing different antifibrotic therapies in vitro.
Figures






References
-
- Liu M, Tanswell K, Post M. Mechanical force-induced signal transduction in lung cells. Am J Physiol Lung Cell Mol Physiol. 1999;277:667–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

