Beta-cell regeneration from vimentin+/MafB+ cells after STZ-induced extreme beta-cell ablation
- PMID: 26129776
- PMCID: PMC4486952
- DOI: 10.1038/srep11703
Beta-cell regeneration from vimentin+/MafB+ cells after STZ-induced extreme beta-cell ablation
Abstract
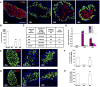
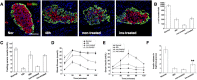
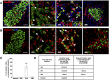
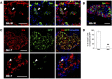
Loss of functional beta-cells is fundamental in both type 1 and type 2 diabetes. In situ beta-cell regeneration therefore has garnered great interest as an approach to diabetes therapy. Here, after elimination of pre-existing beta cells by a single high-dose of streptozotocin (STZ), we demonstrated that a considerable amount of beta-like-cells was generated within 48 hrs. But the newly formed insulin producing cells failed to respond to glucose challenge at this time and diminished afterwards. Insulin treatment to normalize the glucose level protected the neogenic beta-like cells and the islet function was also gradually matured. Strikingly, intermediate cells lacking epithelial marker E-cadherin but expressing mesenchymal cell-specific marker vimentin appeared within 16 hrs following STZ exposure, which served as the major source of insulin-producing cells observed at 24 hrs. Moreover, these intermediate cells strongly expressed alpha-cell-specific marker MafB. In summary, the data presented here identified a novel intermediate cell type as beta-cell progenitors, showing mesenchymal cell feature as well as alpha-cell marker MafB. Our results might have important implications for efforts to stimulate beta-cell regeneration.
Figures

References
-
- Butler A. E. et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52, 102–110 (2003). - PubMed
-
- Renaud Desgraz C. B. a. P. L. H. β-Cell regeneration the pancreatic intrinsic faculty. Trends Endocrinol Metab 22, 34–43 (2010). - PubMed
-
- Zhou Q. & Melton D. A. Extreme makeover: converting one cell into another. Cell Stem Cell 3, 382–388 (2008). - PubMed
-
- Shen J., Cheng Y., Han Q., Mu Y. & Han W. Generating insulin-producing cells for diabetic therapy: existing strategies and new development. Ageing Res Rev 12, 469–478,(2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

