Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species
- PMID: 26129847
- PMCID: PMC4529699
- DOI: 10.1186/s13287-015-0116-z
Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species
Abstract
Introduction: Administration of mesenchymal stem cells (MSCs) represents a promising treatment option for patients suffering from immunological and degenerative disorders. Accumulating evidence indicates that the healing effects of MSCs are mainly related to unique paracrine properties, opening opportunities for secretome-based therapies. Apart from soluble factors, MSCs release functional small RNAs via extracellular vesicles (EVs) that seem to convey essential features of MSCs. Here we set out to characterize the full small RNAome of MSC-produced exosomes.
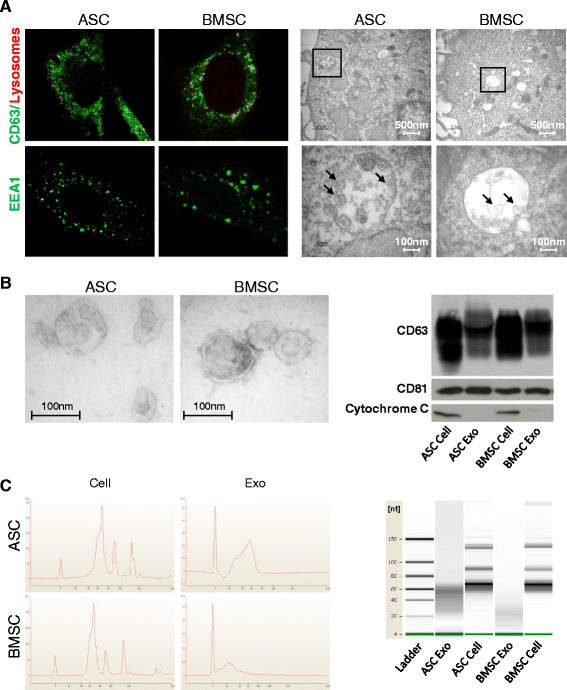
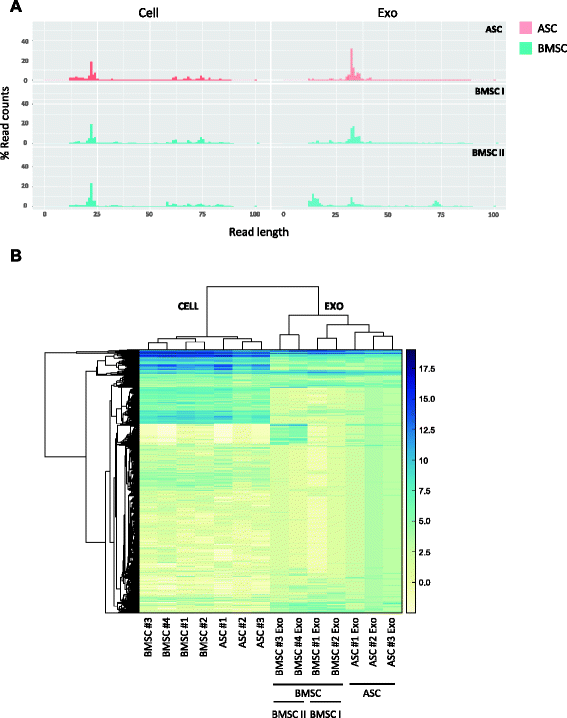
Methods: We set up a protocol for isolating exosomes released by early passage adipose- (ASC) and bone marrow-MSCs (BMSC) and characterized them via electron microscopy, protein analysis and small RNA-sequencing. We developed a bioinformatics pipeline to define the exosome-enclosed RNA species and performed the first complete small RNA characterization of BMSCs and ASCs and their corresponding exosomes in biological replicates.
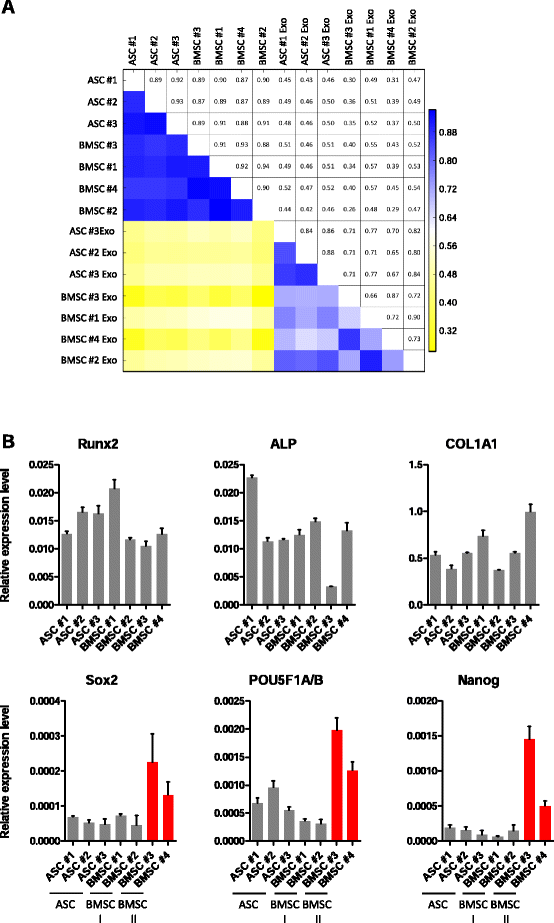
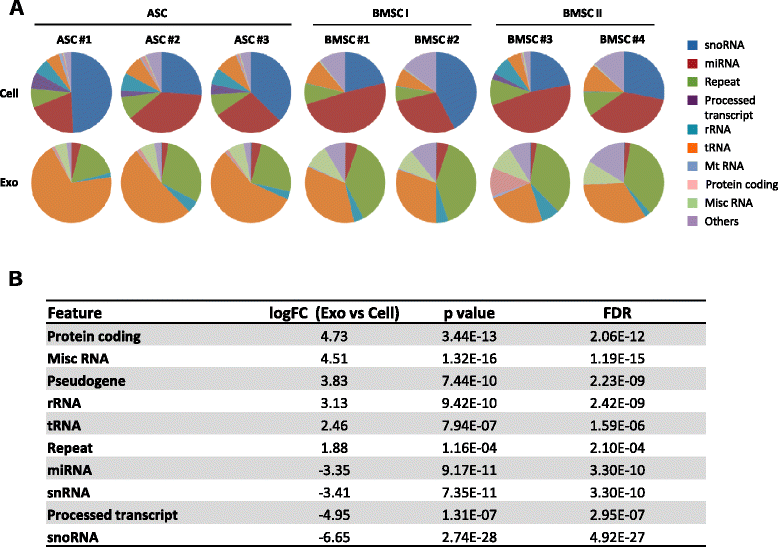
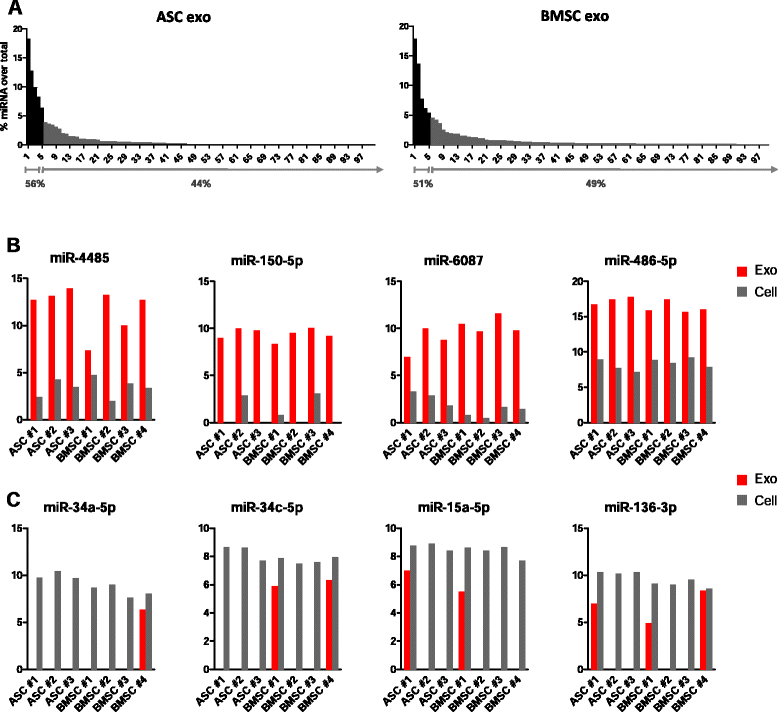
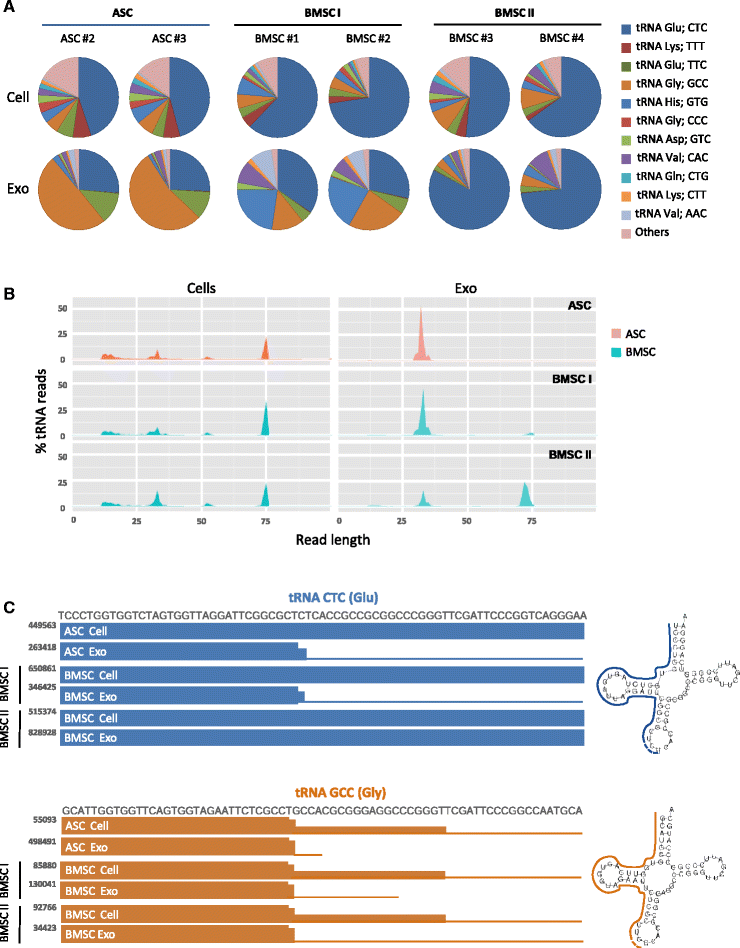
Results: Our analysis revealed that primary ASCs and BMSCs have highly similar small RNA expression profiles dominated by miRNAs and snoRNAs (together 64-71 %), of which 150-200 miRNAs are present at physiological levels. In contrast, the miRNA pool in MSC exosomes is only 2-5 % of the total small RNAome and is dominated by a minor subset of miRNAs. Nevertheless, the miRNAs in exosomes do not merely reflect the cellular content and a defined set of miRNAs are overrepresented in exosomes compared to the cell of origin. Moreover, multiple highly expressed miRNAs are precluded from exosomal sorting, consistent with the notion that these miRNAs are involved in functional repression of RNA targets. While ASC and BMSC exosomes are similar in RNA class distribution and composition, we observed striking differences in the sorting of evolutionary conserved tRNA species that seems associated with the differentiation status of MSCs, as defined by Sox2, POU5F1A/B and Nanog expression.
Conclusions: We demonstrate that primary MSCs release small RNAs via exosomes, which are increasingly implicated in intercellular communications. tRNAs species, and in particular tRNA halves, are preferentially released and their specific sorting into exosomes is related to MSC tissue origin and stemness. These findings may help to understand how MSCs impact neighboring or distant cells with possible consequences for their therapeutic usage.
Figures

References
-
- Johnson A, Dorshkind K. Stromal cells in myeloid and lymphoid long-term bone marrow cultures can support multiple hemopoietic lineages and modulate their production of hemopoietic growth factors. Blood. 1986;68:1348–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

