Enantioselective α-Alkylation of Aldehydes by Photoredox Organocatalysis: Rapid Access to Pharmacophore Fragments from β-Cyanoaldehydes
- PMID: 26130043
- PMCID: PMC4548807
- DOI: 10.1002/anie.201503789
Enantioselective α-Alkylation of Aldehydes by Photoredox Organocatalysis: Rapid Access to Pharmacophore Fragments from β-Cyanoaldehydes
Abstract
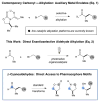
The combination of photoredox catalysis and enamine catalysis has enabled the development of an enantioselective α-cyanoalkylation of aldehydes. This synergistic catalysis protocol allows for the coupling of two highly versatile yet orthogonal functionalities, allowing rapid diversification of the oxonitrile products to a wide array of medicinally relevant derivatives and heterocycles. This methodology has also been applied to the total synthesis of the lignan natural product (-)-bursehernin.
Keywords: aldehydes; alkylation; organocatalysis; photoredox catalysis; total synthesis.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
References
-
- Vesely J, Rios R. Chem Cat Chem. 2012;4:942–953.
-
- Enders D, Eichenauer H. Tetrahedron Lett. 1977;18:191–194.
- Enders D, Eichenauer H. Chem Ber. 1979;112:2933–2960.
- Evans DA, Ennis MD, Mathre DJ. J Am Chem Soc. 1982;104:1737–1739.
- Dolling UH, Davis P, Grabowski EJJ. J Am Chem Soc. 1984;106:446–447.
- Myers AG, Yang BH, Chen H, Gleason JL. J Am Chem Soc. 1994;116:9361–9362.
- Oppolzer W, Moretti R, Thomi S. Tetrahedron Lett. 1989;30:5603–5606.
- Imai M, Hagihara A, Kawasaki H, Manabe K, Koga K. J Am Chem Soc. 1994;116:8829–8830.
- Job A, Janeck CF, Bettray W, Peters R, Enders D. Tetrahedron. 2002;58:2253–2329.
- Doyle AG, Jacobsen EN. J Am Chem Soc. 2005;127:62–63. - PubMed
- Brak K, Jacobsen EN. Angew Chem Int Ed. 2013;52:534–561. - PMC - PubMed
- Angew Chem. 2013;125:558–588.
-
-
For a Suzuki-Miyaura coupling approach using α-bromo amides see: Fischer C, Fu GC. J Am Chem Soc. 2005;127:4594–4595.
-
-
-
MacMillan DWC. Nature. 2008;455:304–308.Mukherjee S, Yang JW, Hoffmann S, List B. Chem Rev. 2007;107:5471–5569.Erkkilä A, Majander I, Pihko PM. Chem Rev. 2007;107:5416–5470.For early reports of aldehyde α-alkylation reactions, see: Vignola N, List B. J Am Chem Soc. 2004;126:450–451.Enders D, Wang C, Bats JW. Angew Chem Int Ed. 2008;47:7539–7542.Angew Chem. 2008;120:7649–7653.List B, Čorić I, OGrygorenko O, Kaib PSJ, Komarov I, Lee A, Leutzsch M, Chandra Pan S, Tymtsunik AV, van Gemmeren M. Angew Chem Int Ed. 2014;53:282–285.Angew Chem. 2014;126:286–289.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources