Indoleamine 2,3-dioxygenase-dependent neurotoxic kynurenine metabolism mediates inflammation-induced deficit in recognition memory
- PMID: 26130057
- PMCID: PMC4631688
- DOI: 10.1016/j.bbi.2015.06.022
Indoleamine 2,3-dioxygenase-dependent neurotoxic kynurenine metabolism mediates inflammation-induced deficit in recognition memory
Abstract
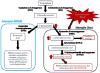
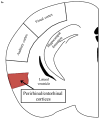
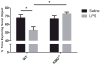
Cognitive dysfunction in depression is a prevalent and debilitating symptom that is poorly treated by the currently available pharmacotherapies. Research over the past decade has provided evidence for proinflammatory involvement in the neurobiology of depressive disorders and symptoms associated with these disorders, including aspects of memory dysfunction. Recent clinical studies implicate inflammation-related changes in kynurenine metabolism as a potential pathogenic factor in the development of a range of depressive symptoms, including deficits in cognition and memory. Additionally, preclinical work has demonstrated a number of mood-related depressive-like behaviors to be dependent on indoleamine 2,3-dioxygenase-1 (IDO1), the inflammation-induced rate-limiting enzyme of the kynurenine pathway. Here, we demonstrate in a mouse model, that peripheral administration of endotoxin induced a deficit in recognition memory. Mice deficient in IDO were protected from cognitive impairment. Furthermore, endotoxin-induced inflammation increased kynurenine metabolism within the perirhinal/entorhinal cortices, brain regions which have been implicated in recognition memory. A single peripheral injection of kynurenine, the metabolic product of IDO1, was sufficient to induce a deficit in recognition memory in both control and IDO null mice. Finally, kynurenine monooxygenase (KMO) deficient mice were also protected from inflammation-induced deficits on novel object recognition. These data implicate IDO-dependent neurotoxic kynurenine metabolism as a pathogenic factor for cognitive dysfunction in inflammation-induced depressive disorders and a potential novel target for the treatment of these disorders.
Keywords: Behavior; Indoleamine 2,3-dioxygenase; Kynurenine; Kynurenine monooxygenase; Mouse; Neuroinflammation; Neuropsychiatric symptom; Novel object recognition; Perirhinal cortex; Recognition memory.
Published by Elsevier Inc.
Conflict of interest statement
None of the authors have any conflicts of interest to disclose
Figures









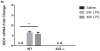
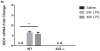
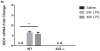
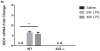
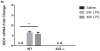




References
-
- Andre C, O’Connor JC, Kelley KW, Lestage J, Dantzer R, Castanon N. Spatio-temporal differences in the profile of murine brain expression of proinflammatory cytokines and indoleamine 2,3-dioxygenase in response to peripheral lipopolysaccharide administration. J Neuroimmunol. 2008;200(1–2):90–99. - PMC - PubMed
-
- Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry. 2004;56(11):819–824. - PubMed
-
- Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002;7(5):468–473. - PubMed
-
- Chiarugi A, Carpenedo R, Moroni F. Kynurenine disposition in blood and brain of mice: effects of selective inhibitors of kynurenine hydroxylase and of kynureninase. J Neurochem. 1996;67(2):692–698. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

