The XRCC1 phosphate-binding pocket binds poly (ADP-ribose) and is required for XRCC1 function
- PMID: 26130715
- PMCID: PMC4538820
- DOI: 10.1093/nar/gkv623
The XRCC1 phosphate-binding pocket binds poly (ADP-ribose) and is required for XRCC1 function
Abstract
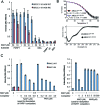
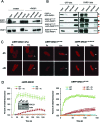
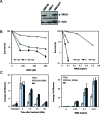
Poly (ADP-ribose) is synthesized at DNA single-strand breaks and can promote the recruitment of the scaffold protein, XRCC1. However, the mechanism and importance of this process has been challenged. To address this issue, we have characterized the mechanism of poly (ADP-ribose) binding by XRCC1 and examined its importance for XRCC1 function. We show that the phosphate-binding pocket in the central BRCT1 domain of XRCC1 is required for selective binding to poly (ADP-ribose) at low levels of ADP-ribosylation, and promotes interaction with cellular PARP1. We also show that the phosphate-binding pocket is required for EGFP-XRCC1 accumulation at DNA damage induced by UVA laser, H2O2, and at sites of sub-nuclear PCNA foci, suggesting that poly (ADP-ribose) promotes XRCC1 recruitment both at single-strand breaks globally across the genome and at sites of DNA replication stress. Finally, we show that the phosphate-binding pocket is required following DNA damage for XRCC1-dependent acceleration of DNA single-strand break repair, DNA base excision repair, and cell survival. These data support the hypothesis that poly (ADP-ribose) synthesis promotes XRCC1 recruitment at DNA damage sites and is important for XRCC1 function.
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- Caldecott K.W. Single-strand break repair and genetic disease. Nat. Rev. Genet. 2008;9:619–631. - PubMed
-
- Caldecott K.W. XRCC1 and DNA strand break repair. DNA Repair (Amst.) 2003;2:955–969. - PubMed
-
- Thompson L.H., West M.G. XRCC1 keeps DNA from getting stranded. Mutat. Res. 2000;459:1–18. - PubMed
-
- Thompson L.H., Brookman K.W., Dillehay L.E., Carrano A.V., Mazrimas J.A., Mooney C.L., Minkler J.L. A CHO-cell strain having hypersensitivity to mutagens, a defect in DNA strand-break repair, and an extraordinary baseline frequency of sister-chromatid exchange. Mutat. Res. 1982;95:427–440. - PubMed
-
- Zdzienicka M.Z., van der Schans G.P., Natarajan A.T., Thompson L.H., Neuteboom I., Simons J.W. A Chinese hamster ovary cell mutant (EM-C11) with sensitivity to simple alkylating agents and a very high level of sister chromatid exchanges. Mutagenesis. 1992;7:265–269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

