Randomized phase 2 study of carboplatin and bevacizumab in recurrent glioblastoma
- PMID: 26130744
- PMCID: PMC4648304
- DOI: 10.1093/neuonc/nov104
Randomized phase 2 study of carboplatin and bevacizumab in recurrent glioblastoma
Abstract
Background: The optimal use of bevacizumab in recurrent glioblastoma (GBM), including the choice of monotherapy or combination therapy, remains uncertain. The purpose of this study was to compare combination therapy with bevacizumab monotherapy.
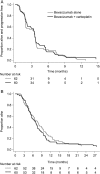
Methods: This was a 2-part randomized phase 2 study. Eligibility criteria included recurrent GBM after radiotherapy and temozolomide, no other chemotherapy for GBM, and Eastern Cooperative Oncology Group performance status 0-2. The primary objective (Part 1) was to determine the effect of bevacizumab plus carboplatin versus bevacizumab monotherapy on progression-free survival (PFS) using modified Response Assessment in Neuro-Oncology criteria. Bevacizumab was given every 2 weeks, 10 mg/kg; and carboplatin every 4 weeks, (AUC 5). On progression, patients able to continue were randomized to continue or cease bevacizumab (Part 2). Secondary endpoints included objective radiological response rate (ORR), quality of life, toxicity, and overall survival (OS).
Results: One hundred twenty-two patients (median age, 55y) were enrolled to Part 1 from 18 Australian sites. Median follow-up was 32 months, and median on-treatment time was 3.3 months. Median PFS was 3.5 months for each arm (hazard ratio [HR]: 0.92, 95% CI: 0.64-1.33, P = .66). ORR was 14% (combination) versus 6% (monotherapy) (P = .18). Median OS was 6.9 (combination) versus 7.5 months (monotherapy) (HR: 1.18, 95% CI: 0.82-1.69, P = .38). The incidence of bevacizumab-related adverse events was similar to prior literature, with no new toxicity signals. Toxicities were higher in the combination arm. Part 2 data (n = 48) will be reported separately.
Conclusions: Adding carboplatin resulted in more toxicity without additional clinical benefit. Clinical outcomes in patients with recurrent GBM treated with bevacizumab were inferior to those in previously reported studies.
Clinical trials registration nr: ACTRN12610000915055.
Keywords: bevacizumab; carboplatin; glioblastoma.
© The Author(s) 2015. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. - PubMed
-
- Schwartzbaum JA, Fisher JL, Aldape KD, et al. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2(9):494–503; quiz 491 p following 516. - PubMed
-
- Australian Cancer Network Adult Brain Tumour Guidelines Working Party, Clinical Practice Guidelines for the Management of Adult Gliomas: Astrocytomas and Oligodendrogliomas. Sydney: Cancer Council Australia, Australian Cancer Network and Clinical Oncological Society of Australia Inc., 2009.
-
- Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17(8):2572–2578. - PubMed
-
- Nieder C, Grosu AL, Molls M. A comparison of treatment results for recurrent malignant gliomas. Cancer Treat Rev. 2000;26(6):397–409. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical