Distinct roles for GABA across multiple timescales in mammalian circadian timekeeping
- PMID: 26130805
- PMCID: PMC4517259
- DOI: 10.1073/pnas.1420753112
Distinct roles for GABA across multiple timescales in mammalian circadian timekeeping
Abstract
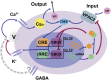
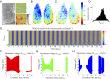
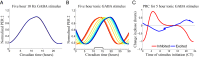
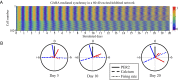
The suprachiasmatic nuclei (SCN), the central circadian pacemakers in mammals, comprise a multiscale neuronal system that times daily events. We use recent advances in graphics processing unit computing to generate a multiscale model for the SCN that resolves cellular electrical activity down to the timescale of individual action potentials and the intracellular molecular events that generate circadian rhythms. We use the model to study the role of the neurotransmitter GABA in synchronizing circadian rhythms among individual SCN neurons, a topic of much debate in the circadian community. The model predicts that GABA signaling has two components: phasic (fast) and tonic (slow). Phasic GABA postsynaptic currents are released after action potentials, and can both increase or decrease firing rate, depending on their timing in the interspike interval, a modeling hypothesis we experimentally validate; this allows flexibility in the timing of circadian output signals. Phasic GABA, however, does not significantly affect molecular timekeeping. The tonic GABA signal is released when cells become very excited and depolarized; it changes the excitability of neurons in the network, can shift molecular rhythms, and affects SCN synchrony. We measure which neurons are excited or inhibited by GABA across the day and find GABA-excited neurons are synchronized by-and GABA-inhibited neurons repelled from-this tonic GABA signal, which modulates the synchrony in the SCN provided by other signaling molecules. Our mathematical model also provides an important tool for circadian research, and a model computational system for the many multiscale projects currently studying brain function.
Keywords: GABA; circadian; mathematical modeling; network; synchronization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

