Structural elucidation of chemical constituents from Benincasa hispida seeds and Carissa congesta roots by gas chromatography: Mass spectroscopy
- PMID: 26130941
- PMCID: PMC4471656
- DOI: 10.4103/0974-8490.157179
Structural elucidation of chemical constituents from Benincasa hispida seeds and Carissa congesta roots by gas chromatography: Mass spectroscopy
Abstract
Background: Benincasa hispida (BH) and Carissa congesta (CC) are regarded as ethnopharmacological imperative plants in Asian countries.
Objective: Phytochemical screening of the extracts has shown the presence of steroids, flavonoids, saponins, glycosides, tannins, phenolic compounds, fixed oils, and fats in the BH and CC extracts. The presence of lupeol has been reported previously by us using high-performance thin-layer chromatography and high-performance liquid chromatography.
Materials and methods: Present research studies encompasses identification of chemical constituents in BH seeds and CC roots petroleum ether extracts by hyphenated technique such as gas chromatography-mass spectroscopy (MS) which when coupled gives a clear insight of constituents.
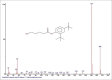
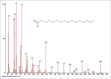
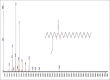
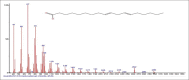
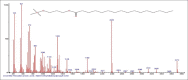
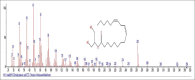
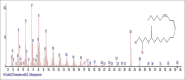
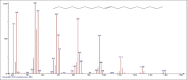
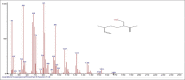
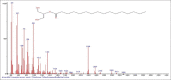
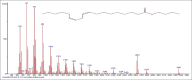
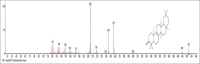
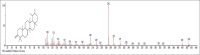
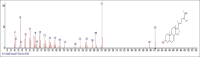
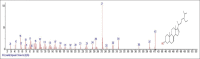
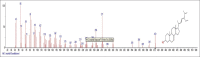
Results: The components were identified by matching mass spectra with MS libraries. There were 13 and 10 different compounds analyzed from CC and BH, respectively. The components present were Pentanoic acid, 5-hydroxy, 2,4-butylphenyl; n-Hexadecanoic acid (Palmitic acid); Sulfurous acid, 2-ethylhexylhepatdecyl ester; n-Tridecane; 6-methyltridecane; (9E, 12E)-9,12-Octadecadienyl chloride, Hexadecanoic acid, 3-(trimethylsilyl)-oxy] propyl ester; 9,12-Octadecadenoic acid, 2 hydroxy-1-(hyroxymethylethyl) ester; 9,12-Octadecadienoic acid, 2,3 dihydroxypropyl ester; n-Propyl-9,12-Octadecadienoate, Lupeol; Taraxasterol; 6a, 14a-Methanopicene, perhydro-12,4a, 61a, 9,9,12a-hepatmethyl-10-hydoxy and 9-Octadecene; 2-Isoprpenyl-5-methyl-6-hepten-1-ol; n-Hexadecanoic acid, 2-hyroxy-1-(hydroxymethyl) ethyl ether; Butyl-9,12-Octadecadieonate; Friedoolean-8-en-3-one; friedours-7-en-3-one; 13,27-Cyclosuran-3-one; Stigmaste-7,25-dien-3-ol (3β, 5α); Stigmasta-7,16-dien-3-ol; chrondrillasterol in BH seeds and CC roots extracts respectively.
Conclusion: Eluted components from the extracts could provide further researchers to work with various pharmacological activities related models and studies.
Keywords: 25-dien-3-ol; Apocynaceae; Benincasa hispida; Carissa congesta; Cururbitaceae; Stigmaste-7; chrondrillasterol; gas chromatography; lupeol; mass spectroscopy.
Conflict of interest statement
Figures










References
-
- Harborne JB. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. 3rd ed. New Delhi, India: Springer (India) Private Limited Publication House; 2005. pp. 1–36.
-
- Iwashina T. The structure and distribution of the flavonoids in plants. J Plant Res. 2000;113:287–99.
-
- Samuelsson G. Drugs of Natural Origin: A Textbook of Pharmacognosy. 5th ed. Sweden, Stockholm: Swedish Pharmaceutical Press; 2004. pp. 154–5.
-
- Newman DJ, Cragg GM, Snader KM. The influence of natural products upon drug discovery. Nat Prod Rep. 2000;17:215–34. - PubMed
-
- Butler MS. The role of natural product chemistry in drug discovery. J Nat Prod. 2004;67:2141–53. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
