Bone fracture and the interaction between bisphosphonates and proton pump inhibitors: a meta-analysis
- PMID: 26131063
- PMCID: PMC4483859
Bone fracture and the interaction between bisphosphonates and proton pump inhibitors: a meta-analysis
Abstract
Objective: Recent studies suggested an increased risk of fractures with interaction between bisphosphonates (BPs) and proton pump inhibitors (PPIs). We performed a meta-analysis of fractures between patients taking BPs/PPIs and those taking BPs only.
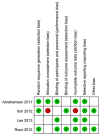
Methods: We conducted a PubMed database and Ovid database search, as well as Cochrane Library search (up to July 2014) for studies assessing the association between fractures and BPs or/and PPIs. We performed random effects meta-analysis of odds ratios (OR) according to fracture type and conducted subgroup analyses by race and BP subtypes. Heterogeneity was assessed using Q statistics and I(2) statistic.
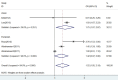
Results: After study selection, 4 unique studies (5 comparisons) including 57259 patients were available for this meta-analysis. Pooled analysis of overall fracture risk of BP+PPI group versus BP group showed a significant increase in risk of fractures (OR = 1.52, P = 0.025), with substantial heterogeneity. However, heterogeneity was drastically reduced in subgroup of Asian (I(2) = 24% and P = 0.251), and fracture risk showed a significant increase (OR = 1.75, P = 0.026). In contrast, heterogeneity was little eliminated in subgroup of European, and fracture risk was no statistical difference (OR = 1.42, P = 0.068). Three studies including 4 comparisons reported on spine fracture were included in the pooled analysis demonstrating an increased spine fracture risk associated with BP/PPI interaction (OR = 1.60, 95% CI 1.13-2.26, P = 0.008, I(2) = 58.6%).
Conclusions: This meta-analysis suggests that there is an interaction associated with increased fracture risk (particularly for spine and Asian race) between BP and PPI use. Clinicians should carefully evaluate such risk factors for osteoporosis in patients taking BPs, before routinely prescribing PPIs, and make a careful judgment as to whether PPIs may be safe for patients at high risk of fractures.
Keywords: Fracture; bisphosphonate; meta-analysis; proton pump inhibitor.
Figures






Similar articles
-
Meta-analysis: risk of fractures with acid-suppressing medication.Bone. 2011 Apr 1;48(4):768-76. doi: 10.1016/j.bone.2010.12.015. Epub 2010 Dec 23. Bone. 2011. PMID: 21185417
-
Proton-pump inhibitors and risk of fractures: an update meta-analysis.Osteoporos Int. 2016 Jan;27(1):339-47. doi: 10.1007/s00198-015-3365-x. Osteoporos Int. 2016. PMID: 26462494
-
Proton pump inhibitors therapy and risk of bone diseases: An update meta-analysis.Life Sci. 2019 Feb 1;218:213-223. doi: 10.1016/j.lfs.2018.12.058. Epub 2018 Dec 31. Life Sci. 2019. PMID: 30605646
-
Acid-Suppressive Drugs and Risk of Fracture in Children and Young Adults: A Meta-Analysis of Observational Studies.Front Pharmacol. 2021 Aug 6;12:712939. doi: 10.3389/fphar.2021.712939. eCollection 2021. Front Pharmacol. 2021. PMID: 34421609 Free PMC article. Review.
-
Proton pump inhibitors' use and risk of hip fracture: a systematic review and meta-analysis.Rheumatol Int. 2018 Nov;38(11):1999-2014. doi: 10.1007/s00296-018-4142-x. Epub 2018 Aug 29. Rheumatol Int. 2018. PMID: 30159775
Cited by
-
Surgical Procedures Used for Correction of Scheuermann's Kyphosis: A Meta-Analysis.Pain Res Manag. 2021 Oct 23;2021:2142964. doi: 10.1155/2021/2142964. eCollection 2021. Pain Res Manag. 2021. PMID: 34725561 Free PMC article. Review.
-
Association between proton pump inhibitor use and risk of fracture: A population-based case-control study.PLoS One. 2020 Jul 30;15(7):e0235163. doi: 10.1371/journal.pone.0235163. eCollection 2020. PLoS One. 2020. PMID: 32730257 Free PMC article.
-
Proton Pump Inhibitor Use Affects Pseudarthrosis Rates and Influences Patient-Reported Outcomes.Global Spine J. 2020 Feb;10(1):55-62. doi: 10.1177/2192568219853222. Epub 2019 Jun 10. Global Spine J. 2020. PMID: 32002350 Free PMC article.
-
Proton Pump Inhibitors and Fractures in Adults: A Critical Appraisal and Review of the Literature.Int J Endocrinol. 2021 Jan 15;2021:8902367. doi: 10.1155/2021/8902367. eCollection 2021. Int J Endocrinol. 2021. PMID: 33510787 Free PMC article. Review.
-
Acid-Suppressive Therapy and Risk of Infections: Pros and Cons.Clin Drug Investig. 2017 Jul;37(7):587-624. doi: 10.1007/s40261-017-0519-y. Clin Drug Investig. 2017. PMID: 28361440 Review.
References
-
- Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–22. - PubMed
-
- Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, Nevitt MC, Suryawanshi S, Cummings SR. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab. 2000;85:4118–24. - PubMed
-
- Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH 3rd, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344–52. - PubMed
LinkOut - more resources
Full Text Sources
