Quality and Quantity of Published Studies Evaluating Lumbar Fusion during the Past 10 Years: A Systematic Review
- PMID: 26131387
- PMCID: PMC4472285
- DOI: 10.1055/s-0035-1552984
Quality and Quantity of Published Studies Evaluating Lumbar Fusion during the Past 10 Years: A Systematic Review
Abstract
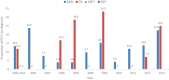
Study Design Systematic review. Clinical Questions (1) Has the proportion and number of randomized controlled trials (RCTs) as an indicator of quality of evidence regarding lumbar fusion increased over the past 10 years? (2) Is there a difference in the proportion of RCTs among the four primary fusion diagnoses (degenerative disk disease, spondylolisthesis, deformity, and adjacent segment disease) over the past 10 years? (3) Is there a difference in the type and quality of clinical outcomes measures reported among RCTs over time? (4) Is there a difference in the type and quality of adverse events measures reported among RCTs over time? (5) Are there changes in fusion surgical approach and techniques over time by diagnosis over the past 10 years? Methods Electronic databases and reference lists of key articles were searched from January 1, 2004, through December 31, 2013, to identify lumbar fusion RCTs. Fusion studies designed specifically to evaluate recombinant human bone morphogenetic protein-2 or other bone substitutes, revision surgery studies, nonrandomized comparison studies, case reports, case series, and cost-effectiveness studies were excluded. Results Forty-two RCTs between January 1, 2004, and December 31, 2013, met the inclusion criteria and form the basis for this report. There were 35 RCTs identified evaluating patients diagnosed with degenerative disk disease, 4 RCTs evaluating patients diagnosed with degenerative spondylolisthesis, and 3 RCTs evaluating patients with a combination of degenerative disk disease and degenerative spondylolisthesis. No RCTs were identified evaluating patients with deformity or adjacent segment disease. Conclusions This structured review demonstrates that there has been an increase in the available clinical database of RCTs using patient-reported outcomes evaluating the benefit of lumbar spinal fusion for the diagnoses of degenerative disk disease and degenerative spondylolisthesis. Gaps remain in the standardization of reportage of adverse events in such trials, as well as uniformity of surgical approaches used. Finally, continued efforts to develop higher-quality data for other surgical indications for lumbar fusion, most notably in the presence of adult spinal deformity and revision of prior surgical fusions, appear warranted.
Keywords: adverse events; evidence-based medicine; lumbar spine; spinal fusion; spine surgery.
Conflict of interest statement
Figures



References
-
- Anderson P A, Hart R A. Adverse events recording and reporting in clinical trials of cervical total disk replacement. Instr Course Lect. 2014;63:287–296. - PubMed
-
- Aoki Y, Yamagata M, Ikeda Y. et al.A prospective randomized controlled study comparing transforaminal lumbar interbody fusion techniques for degenerative spondylolisthesis: unilateral pedicle screw and 1 cage versus bilateral pedicle screws and 2 cages. J Neurosurg Spine. 2012;17(2):153–159. - PubMed
-
- Auerbach J D, Jones K J, Milby A H, Anakwenze O A, Balderston R A. Segmental contribution toward total lumbar range of motion in disc replacement and fusions: a comparison of operative and adjacent levels. Spine (Phila Pa 1976) 2009;34(23):2510–2517. - PubMed
-
- Berg S, Fritzell P, Tropp H. Sex life and sexual function in men and women before and after total disc replacement compared with posterior lumbar fusion. Spine J. 2009;9(12):987–994. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

