Two Distinct Cdc2 Pools Regulate Cell Cycle Progression and the DNA Damage Response in the Fission Yeast S.pombe
- PMID: 26131711
- PMCID: PMC4488491
- DOI: 10.1371/journal.pone.0130748
Two Distinct Cdc2 Pools Regulate Cell Cycle Progression and the DNA Damage Response in the Fission Yeast S.pombe
Abstract
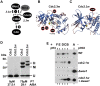
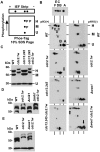
The activity of Cdc2 (CDK1) kinase, which coordinates cell cycle progression and DNA break repair, is blocked upon its phosphorylation at tyrosine 15 (Y15) by Wee1 kinase in the presence of DNA damage. How Cdc2 can support DNA repair whilst being inactivated by the DNA damage checkpoint remains to be explained. Human CDK1 is phosphorylated by Myt1 kinase at threonine 14 (T14) close to its ATP binding site before being modified at threonine 161 (T167Sp) in its T-loop by the CDK-activating kinase (CAK). While modification of T161 promotes association with the cyclin partner, phosphorylation of T14 inhibits the CDK1-cyclin complex. This inhibition is further enforced by the modification of Y15 by Wee1 in the presence of DNA lesions. In S.pombe, the dominant inhibition of Cdc2 is provided by the phosphorylation of Y15 and only a small amount of Cdc2 is modified at T14 when cells are in S phase. Unlike human cells, both inhibitory modifications are executed by Wee1. Using the novel IEFPT technology, which combines isoelectric focusing (IEF) with Phos-tag SDS electrophoresis (PT), we report here that S.pombe Cdc2 kinase exists in seven forms. While five forms are phosphorylated, two species are not. Four phospho-forms associate with cyclin B (Cdc13) of which only two are modified at Y15 by Wee1. Interestingly, only one Y15-modified species carries also the T14 modification. The fifth phospho-form has a low affinity for cyclin B and is neither Y15 nor T14 modified. The two unphosphorylated forms may contribute directly to the DNA damage response as only they associate with the DNA damage checkpoint kinase Chk1. Interestingly, cyclin B is also present in the unphosphorylated pool. We also show that the G146D mutation in Cdc2.1w, which renders Cdc2 insensitive to Wee1 inhibition, is aberrantly modified in a Wee1-dependent manner. In conclusion, our work adds support to the idea that two distinct Cdc2 pools regulate cell cycle progression and the response to DNA damage.
Conflict of interest statement
Figures


Similar articles
-
The Wee1 protein kinase regulates T14 phosphorylation of fission yeast Cdc2.Mol Biol Cell. 1995 Apr;6(4):371-85. doi: 10.1091/mbc.6.4.371. Mol Biol Cell. 1995. PMID: 7626804 Free PMC article.
-
Chk1 is a wee1 kinase in the G2 DNA damage checkpoint inhibiting cdc2 by Y15 phosphorylation.EMBO J. 1997 Feb 3;16(3):545-54. doi: 10.1093/emboj/16.3.545. EMBO J. 1997. PMID: 9034337 Free PMC article.
-
Coupling of T161 and T14 phosphorylations protects cyclin B-CDK1 from premature activation.Mol Biol Cell. 2011 Nov;22(21):3971-85. doi: 10.1091/mbc.E11-02-0136. Epub 2011 Sep 7. Mol Biol Cell. 2011. PMID: 21900495 Free PMC article.
-
Regulation of Cdc2 activity by phosphorylation at T14/Y15.Prog Cell Cycle Res. 1996;2:99-105. doi: 10.1007/978-1-4615-5873-6_10. Prog Cell Cycle Res. 1996. PMID: 9552387 Review.
-
Establishment of and recovery from damage checkpoint requires sequential interactions of Crb2 with protein kinases Rad3, Chk1, and Cdc2.Cold Spring Harb Symp Quant Biol. 2000;65:443-9. doi: 10.1101/sqb.2000.65.443. Cold Spring Harb Symp Quant Biol. 2000. PMID: 12760060 Review. No abstract available.
Cited by
-
The cyclin-dependent kinase G group defines a thermo-sensitive alternative splicing circuit modulating the expression of Arabidopsis ATU2AF65A.Plant J. 2018 Jun;94(6):1010-1022. doi: 10.1111/tpj.13914. Epub 2018 May 10. Plant J. 2018. PMID: 29602264 Free PMC article.
-
Communication between Cyclin-dependent kinase Cdc2 and the Wis1-Spc1 MAPK pathway determines mitotic timing in Schizosaccharomyces pombe.Biol Open. 2020 Jul 21;9(7):bio053322. doi: 10.1242/bio.053322. Biol Open. 2020. PMID: 32554481 Free PMC article.
-
Preclinical Efficacy and Involvement of AKT, mTOR, and ERK Kinases in the Mechanism of Sulforaphane against Endometrial Cancer.Cancers (Basel). 2020 May 18;12(5):1273. doi: 10.3390/cancers12051273. Cancers (Basel). 2020. PMID: 32443471 Free PMC article.
-
Kinome Expansion in the Fusarium oxysporum Species Complex Driven by Accessory Chromosomes.mSphere. 2018 Jun 13;3(3):e00231-18. doi: 10.1128/mSphere.00231-18. Print 2018 Jun 27. mSphere. 2018. PMID: 29898984 Free PMC article.
-
Caffeine Stabilises Fission Yeast Wee1 in a Rad24-Dependent Manner but Attenuates Its Expression in Response to DNA Damage.Microorganisms. 2020 Sep 30;8(10):1512. doi: 10.3390/microorganisms8101512. Microorganisms. 2020. PMID: 33008060 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

