Effects of Infant Pneumococcal Conjugate Vaccination on Serotype Distribution in Invasive Pneumococcal Disease among Children and Adults in Germany
- PMID: 26132078
- PMCID: PMC4488910
- DOI: 10.1371/journal.pone.0131494
Effects of Infant Pneumococcal Conjugate Vaccination on Serotype Distribution in Invasive Pneumococcal Disease among Children and Adults in Germany
Abstract
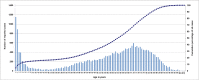
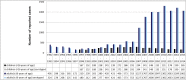
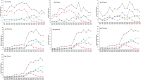
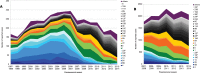
This study describes the effects of the introduction of universal infant pneumococcal conjugate vaccination in 2006 on invasive pneumococcal disease (IPD) among children and adults in Germany with a focus on the dynamics of serotype distribution in vaccinated and non-vaccinated age groups. Over a period of 22 years (1992-2014), microbiological diagnostic laboratories from all over Germany have been sending isolates of IPD cases to the German National Reference Center for Streptococci on a voluntary basis. Streptococcus pneumoniae isolates were serotyped using Neufeld's Quellung method. Among children <16 years, the proportion of PCV7 serotypes among isolates from IPD cases decreased from 61.8% before vaccination (1997-2006) to 23.5% in the early vaccination period (2007-2010; p = 1.30E-72) and sank further to 5.2% in the late vaccination period (2010-2014; p = 4.59E-25). Similar reductions were seen for the separate age groups <2 years, 2-4 years and 5-15 years. Among adults, the proportion of PCV7 serotypes decreased from 43.4% in the pre-vaccination period (1992-2006) to 24.7% (p = 3.78E-88) in the early vaccination period and 8.2% (p = 5.97E-161) in the late vaccination period. Both among children and among adults, the non-PCV7 serotypes 1, 3, 7F and 19A significantly increased in the early vaccination period. After the switch from PCV7 to PVC10/PCV13 for infant vaccination in 2010, serotypes 1, 6A and 7F significantly decreased. A decrease in serotype 19A was only observed in 2013-2014, as compared to 2010-2011 (children p = 4.16E-04, adults p = 6.98E-06). Among adults, serotype 3, which strongly increased in the early vaccination period (p = 4.44E-15), remained at a constant proportion in the late vaccination period. The proportion of non-PCV13 vaccine serotypes increased over the whole vaccination period, with serotypes 10A, 12F, 23B, 24F and 38 most significantly increasing among children and serotypes 6C, 12F, 15A, 22F and 23B increasing among adults. Eight years of childhood pneumococcal conjugate vaccination have had a strong effect on the pneumococcal population in Germany, both among the target group for vaccination as well as among older children and adults.
Conflict of interest statement
Figures
Similar articles
-
Increase of serotypes 15A and 23B in IPD in Germany in the PCV13 vaccination era.BMC Infect Dis. 2015 May 5;15:207. doi: 10.1186/s12879-015-0941-9. BMC Infect Dis. 2015. PMID: 25940580 Free PMC article.
-
Impact of the pneumococcal conjugate vaccines on invasive pneumococcal disease in France, 2001-2012.Vaccine. 2015 Jan 3;33(2):359-66. doi: 10.1016/j.vaccine.2014.11.011. Epub 2014 Nov 20. Vaccine. 2015. PMID: 25448105
-
Impact of pneumococcal conjugate vaccine (PCV7 and PCV13) on pneumococcal invasive diseases in Italian children and insight into evolution of pneumococcal population structure.Vaccine. 2017 Aug 16;35(35 Pt B):4587-4593. doi: 10.1016/j.vaccine.2017.07.010. Epub 2017 Jul 14. Vaccine. 2017. PMID: 28716556
-
Pneumococcal serotype evolution in Western Europe.BMC Infect Dis. 2015 Oct 14;15:419. doi: 10.1186/s12879-015-1147-x. BMC Infect Dis. 2015. PMID: 26468008 Free PMC article. Review.
-
Streptococcus pneumoniae serotype 19A: worldwide epidemiology.Expert Rev Vaccines. 2017 Oct;16(10):1007-1027. doi: 10.1080/14760584.2017.1362339. Epub 2017 Aug 28. Expert Rev Vaccines. 2017. PMID: 28783380 Review.
Cited by
-
Probiotics and carriage of Streptococcus pneumoniae serotypes in Danish children, a double-blind randomized controlled trial.Sci Rep. 2018 Oct 15;8(1):15258. doi: 10.1038/s41598-018-33583-9. Sci Rep. 2018. PMID: 30323328 Free PMC article. Clinical Trial.
-
Pre-vaccine serotype composition within a lineage signposts its serotype replacement - a carriage study over 7 years following pneumococcal conjugate vaccine use in the UK.Microb Genom. 2017 Jun 9;3(6):e000119. doi: 10.1099/mgen.0.000119. eCollection 2017 Jun 30. Microb Genom. 2017. PMID: 29026652 Free PMC article.
-
Systematic review on serotypes distribution of pneumococcal pneumonia in adults and the elderly.BMC Public Health. 2025 Mar 29;25(1):1194. doi: 10.1186/s12889-025-22164-x. BMC Public Health. 2025. PMID: 40158111 Free PMC article.
-
Multi-Valent Protein Hybrid Pneumococcal Vaccines: A Strategy for the Next Generation of Vaccines.Vaccines (Basel). 2021 Mar 2;9(3):209. doi: 10.3390/vaccines9030209. Vaccines (Basel). 2021. PMID: 33801372 Free PMC article. Review.
-
Pivotal Phase 3 Randomized Clinical Trial of the Safety, Tolerability, and Immunogenicity of 20-Valent Pneumococcal Conjugate Vaccine in Adults Aged ≥18 Years.Clin Infect Dis. 2022 Aug 31;75(3):390-398. doi: 10.1093/cid/ciab990. Clin Infect Dis. 2022. PMID: 34940806 Free PMC article. Clinical Trial.
References
-
- Pneumococcal conjugate vaccine for childhood immunization—WHO position paper. Wkly Epidemiol Rec. 2007;82(12):93–104. Epub 2007/03/27. . - PubMed
-
- Le Polain de Waroux O, Flasche S, Prieto-Merino D, Edmunds WJ. Age-dependent prevalence of nasopharyngeal carriage of streptococcus pneumoniae before conjugate vaccine introduction: a prediction model based on a meta-analysis. PLoS One. 2014;9(1):e86136 10.1371/journal.pone.0086136 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

