CFH Y402H and ARMS2 A69S Polymorphisms and Oral Supplementation with Docosahexaenoic Acid in Neovascular Age-Related Macular Degeneration Patients: The NAT2 Study
- PMID: 26132079
- PMCID: PMC4489493
- DOI: 10.1371/journal.pone.0130816
CFH Y402H and ARMS2 A69S Polymorphisms and Oral Supplementation with Docosahexaenoic Acid in Neovascular Age-Related Macular Degeneration Patients: The NAT2 Study
Abstract
Purpose: Genetic susceptibility could be modified by environmental factors and may also influence differential responses to treatments for age-related macular degeneration (AMD). We investigated whether genotype could influence response to docosahexaenoic acid (DHA)-supplementation in the occurrence of choroidal new vessels (CNV).
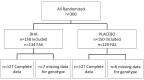
Methods: The Nutritional AMD Treatment 2 (NAT2) study was a randomized, placebo-controlled, double-blind, parallel, comparative study, including 250 patients aged 55 to 85 years with early lesions of age-related maculopathy, visual acuity better than 0.4 Logarithm of Minimum Angle of Resolution units in the study eye and neovascular AMD in the fellow eye. Patients were randomized at baseline to receive either 3 daily fish-oil capsules, each containing 280 mg DHA, 90 mg EPA and 2 mg Vitamin E, or placebo.
Results: Patients carrying the risk allele (C) for CFH Y402H had no statistically significant increased risk for developing CNV in the study eye (Hazard Ratio (HR)=0.97; 95% Confidence Interval (CI): 0.54-1.76 for heterozygous and HR=1.29; 95%CI: 0.69-2.40 for homozygous). Patients carrying the risk allele (T) for ARMS2 A69S had no statistically significant increased risk for developing CNV in the study eye (HR=1.68; 95%CI: 0.91-3.12) for heterozygous and HR=1.78; 95%CI: 0.90-3.52 for homozygous). A significant interaction was observed between CFH Y402H and DHA-supplementation (p=0.01). We showed a protective effect of DHA-supplementation among homozygous non-risk patients. Among these patients, occurrence of CNV was 38.2% in placebo group versus 16.7% in DHA group (p=0.008).
Conclusions: These results suggest that a genetic predisposition to AMD conferred by the CFH Y402H variant limits the benefit provided by DHA supplementation.
Trial registration: ISRCTN registry 98246501.
Conflict of interest statement
Figures
References
-
- Maller J, George S, Purcell S, Fagerness J, Altshuler D, Daly MJ, et al. Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age-related macular degeneration. Nat Genet. 2006;38(9):1055–9. Epub 2006/08/29. doi: ng1873 [pii] 10.1038/ng1873 . - DOI - PubMed
-
- Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102(20):7227–32. Epub 2005/05/05. 10.1073/pnas.0501536102 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

