Hurdles to clinical translation of human induced pluripotent stem cells
- PMID: 26132109
- PMCID: PMC4563685
- DOI: 10.1172/JCI80575
Hurdles to clinical translation of human induced pluripotent stem cells
Abstract
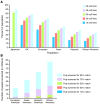
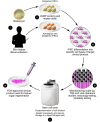
Human pluripotent stem cells are known to have the capacity to renew indefinitely, being intrinsically able to differentiate into many different cell types. These characteristics have generated tremendous enthusiasm about the potential applications of these cells in regenerative medicine. However, major challenges remain with the development and testing of novel experimental stem cell therapeutics in the field. In this Review, we focus on the nature of the preclinical challenges and discuss potential solutions that could help overcome them. Furthermore, we discuss the use of allogeneic versus autologous stem cell products, including a review of their respective advantages and disadvantages, major clinical requirements, quality standards, time lines, and costs of clinical grade development.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

