Use of Time-Resolved Fluorescence to Monitor Bioactive Compounds in Plant Based Foodstuffs
- PMID: 26132136
- PMCID: PMC4600163
- DOI: 10.3390/bios5030367
Use of Time-Resolved Fluorescence to Monitor Bioactive Compounds in Plant Based Foodstuffs
Abstract
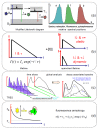
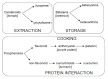
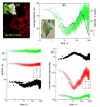
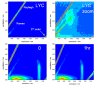
The study of compounds that exhibit antioxidant activity has recently received much interest in the food industry because of their potential health benefits. Most of these compounds are plant based, such as polyphenolics and carotenoids, and there is a need to monitor them from the field through processing and into the body. Ideally, a monitoring technique should be non-invasive with the potential for remote capabilities. The application of the phenomenon of fluorescence has proved to be well suited, as many plant associated compounds exhibit fluorescence. The photophysical behaviour of fluorescent molecules is also highly dependent on their microenvironment, making them suitable probes to monitor changes in pH, viscosity and polarity, for example. Time-resolved fluorescence techniques have recently come to the fore, as they offer the ability to obtain more information, coupled with the fact that the fluorescence lifetime is an absolute measure, while steady state just provides relative and average information. In this work, we will present illustrative time-resolved measurements, rather than a comprehensive review, to show the potential of time-resolved fluorescence applied to the study of bioactive substances. The aim is to help assess if any changes occur in their form, going from extraction via storage and cooking to the interaction with serum albumin, a principal blood transport protein.
Keywords: anthocyanin; betalain; chlorophyll; curcumin; fluorescence lifetime; lycopene.
Figures

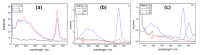
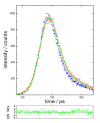
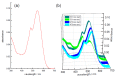
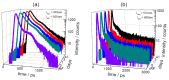
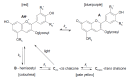

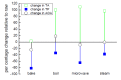

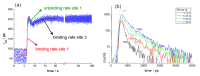
References
-
- Thompson M.D., Thompson H.J., McGinley J.N., Neil E.S., Rush D.K., Holm D.G., Stushnoff C. Functional food characteristics of potato cultivars (Solanum tuberosum L.). Phytochemical composition and inhibition of 1-methyl-1-nitrosourea induced breast cancer in rats. J. Food Comp. Anal. 2009;22:571–576. doi: 10.1016/j.jfca.2008.09.002. - DOI
-
- Dziri S., Hassen I., Fatnassi S., Mrabet Y., Casabianca H., Hanchi B., Hosni K. Phenolic constituents, antioxidant and antimicrobial activities of rosy garlic (Allium roseum var. odoratissimum) J. Funct. Foods. 2012;4:423–432. doi: 10.1016/j.jff.2012.01.010. - DOI
-
- Sancho R.A.S., Pastore G.M. Evaluation of the effects of anthocyanins in type 2 diabetes. Rev. Food Res. Int. 2012;46:378–386. doi: 10.1016/j.foodres.2011.11.021. - DOI
-
- Negi P.S., Namitha K.K. Chemistry and biotechnology of carotenoids. Crit. Rev. Food Sci. Nutr. 2010;50:728–760. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

