Microwave Accelerated Green Synthesis of Stable Silver Nanoparticles with Eucalyptus globulus Leaf Extract and Their Antibacterial and Antibiofilm Activity on Clinical Isolates
- PMID: 26132199
- PMCID: PMC4489395
- DOI: 10.1371/journal.pone.0131178
Microwave Accelerated Green Synthesis of Stable Silver Nanoparticles with Eucalyptus globulus Leaf Extract and Their Antibacterial and Antibiofilm Activity on Clinical Isolates
Abstract
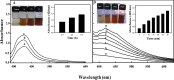
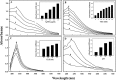
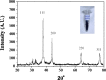
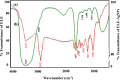
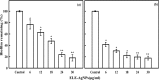
A simple and rapid microwave assisted method of green synthesis of silver nanoparticles (AgNPs) was developed using aqueous leaf extract of Eucalyptus globulus(ELE), and their antibacterial and antibiofilm potential investigated. With this aim, the aqueous solutions of ELE and AgNO3(1 mM) were mixed (1:4 v/v), and microwave irradiated at 2450 Mhz, for 30 sec. The instant color change of the ELE-AgNO3 mixture from pale yellow to dark brown indicated ELE-AgNPs synthesis. The intensity of peak at 428 nm in UV-Vis spectra, due to the surface plasmon resonance of AgNPs, varied with the amount of ELE, AgNO3 concentration, pH and time of incubation. The biosynthesized ELE-AgNPs were characterized by UV-visible spectroscopy, XRD, TEM, SEM-EDX, FTIR and TGA analyses. The size of ELE-AgNPs was determined to be in range of 1.9-4.3 nm and 5-25 nm, with and without microwave treatment, respectively. SEM exhibited the capping of AgNPs with the ELE constituents, and validated by FTIR analysis. The FTIR data revealed the presence of plant organic constituents and metabolites bound to ELE-AgNPs, which contributes for their stability. The antimicrobial activity of ELE-AgNPs was assessed by growth and biofilm inhibition of extended spectrum β-lactamase (ESBL) producing Pseudomonas aeruginosa, Escherichia coli and methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-sensitive Staphylococcus aureus (MSSA) clinical bacterial isolates. The results demonstrated that S. aureus were more sensitive to ELE-AgNPs than E. coli and P. aeruginosa. MRSA exhibited higher sensitive than MSSA, whereas P. aeruginosa were more sensitive than E. coli to ELE-AgNPs treatment. Also, significant (83 ± 3% and 84 ± 5%) biofilm inhibition was observed in case of S. aureus and P. aeruginosa, respectively. The results elucidated environmentally friendly, economical and quick method for production of colloidal bio-functionalized ELE-AgNPs, for effectual clinical applications, as broad spectrum antibacterial agents and biofilm inhibitors.
Conflict of interest statement
Figures





References
-
- Jha AK, Prasad K, Kulkarni AR (2009) Plant system: nature’s nanofactory. Colloids Surf B Biointer 73: 219–223. - PubMed
-
- Annamalai N, Thavasi R, Vijayalakshmi S, Balasubramanian T (2011) A novel thermostable and halostable carboxymethylcellulase from marine bacterium Bacillus licheniformis AU0, World J Microbiol and Biotechnol 27: 2111–2115.
-
- Wilcoxon JP, Abramsb BL (2006) Synthesis, structure and properties of metal nanoclusters. Chem Soc Rev 35: 1162–1194 - PubMed
-
- Alarcon EI, Udekwu K, Skog M (2012) The biocompatibility and antibacterial properties of collagen-stabilized, photochemically prepared silver nanoparticles. Biomater 33:4947−4956. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

