B-Lymphocyte Depletion in Myalgic Encephalopathy/ Chronic Fatigue Syndrome. An Open-Label Phase II Study with Rituximab Maintenance Treatment
- PMID: 26132314
- PMCID: PMC4488509
- DOI: 10.1371/journal.pone.0129898
B-Lymphocyte Depletion in Myalgic Encephalopathy/ Chronic Fatigue Syndrome. An Open-Label Phase II Study with Rituximab Maintenance Treatment
Abstract
Background: Myalgic Encephalopathy/Chronic Fatigue Syndrome (ME/CFS) is a disease of unknown etiology. We previously reported a pilot case series followed by a small, randomized, placebo-controlled phase II study, suggesting that B-cell depletion using the monoclonal anti-CD20 antibody rituximab can yield clinical benefit in ME/CFS.
Methods: In this single-center, open-label, one-armed phase II study (NCT01156909), 29 patients were included for treatment with rituximab (500 mg/m2) two infusions two weeks apart, followed by maintenance rituximab infusions after 3, 6, 10 and 15 months, and with follow-up for 36 months.
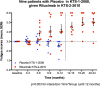
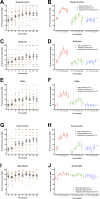
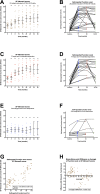
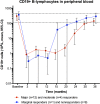
Findings: Major or moderate responses, predefined as lasting improvements in self-reported Fatigue score, were detected in 18 out of 29 patients (intention to treat). Clinically significant responses were seen in 18 out of 28 patients (64%) receiving rituximab maintenance treatment. For these 18 patients, the mean response durations within the 156 weeks study period were 105 weeks in 14 major responders, and 69 weeks in four moderate responders. At end of follow-up (36 months), 11 out of 18 responding patients were still in ongoing clinical remission. For major responders, the mean lag time from first rituximab infusion until start of clinical response was 23 weeks (range 8-66). Among the nine patients from the placebo group in the previous randomized study with no significant improvement during 12 months follow-up after saline infusions, six achieved a clinical response before 12 months after rituximab maintenance infusions in the present study. Two patients had an allergic reaction to rituximab and two had an episode of uncomplicated late-onset neutropenia. Eight patients experienced one or more transient symptom flares after rituximab infusions. There was no unexpected toxicity.
Conclusion: In a subgroup of ME/CFS patients, prolonged B-cell depletion with rituximab maintenance infusions was associated with sustained clinical responses. The observed patterns of delayed responses and relapse after B-cell depletion and regeneration, a three times higher disease prevalence in women than in men, and a previously demonstrated increase in B-cell lymphoma risk for elderly ME/CFS patients, suggest that ME/CFS may be a variant of an autoimmune disease.
Trial registration: ClinicalTrials.gov NCT01156909.
Conflict of interest statement
Figures


References
-
- Carruthers BM, Jain AK, De Meirleir KL, Peterson DL, Klimas NG, Lerner AM, et al. (2003) Myalgic encephalomyelitis/ chronic fatigue syndrome: clinical working case definition, diagnostic and treatment protocols. J Chronic Fatigue Syndr 11: 7–36.
-
- Buchwald D, Pearlman T, Umali J, Schmaling K, Katon W (1996) Functional status in patients with chronic fatigue syndrome, other fatiguing illnesses, and healthy individuals. Am J Med 101: 364–370. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

