High-Fat, High-Calorie Diet Enhances Mammary Carcinogenesis and Local Inflammation in MMTV-PyMT Mouse Model of Breast Cancer
- PMID: 26132316
- PMCID: PMC4586761
- DOI: 10.3390/cancers7030828
High-Fat, High-Calorie Diet Enhances Mammary Carcinogenesis and Local Inflammation in MMTV-PyMT Mouse Model of Breast Cancer
Abstract
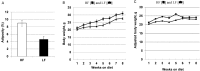
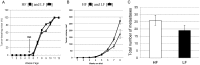
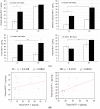
Epidemiological studies provide strong evidence that obesity and the associated adipose tissue inflammation are risk factors for breast cancer; however, the molecular mechanisms are poorly understood. We evaluated the effect of a high-fat/high-calorie diet on mammary carcinogenesis in the immunocompetent MMTV-PyMT murine model. Four-week old female mice (20/group) were randomized to receive either a high-fat (HF; 60% kcal as fat) or a low-fat (LF; 16% kcal) diet for eight weeks. Body weights were determined, and tumor volumes measured by ultrasound, each week. At necropsy, the tumors and abdominal visceral fat were weighed and plasma collected. The primary mammary tumors, adjacent mammary fat, and lungs were preserved for histological and immunohistochemical examination and quantification of infiltrating macrophages, crown-like structure (CLS) formation, and microvessel density. The body weight gains, visceral fat weights, the primary mammary tumor growth rates and terminal weights, were all significantly greater in the HF-fed mice. Adipose tissue inflammation in the HF group was indicated by hepatic steatosis, pronounced macrophage infiltration and CLS formation, and elevations in plasma monocyte chemoattractant protein-1 (MCP-1), leptin and proinflammatory cytokine concentrations. HF intake was also associated with higher tumor-associated microvascular density and the proangiogenic factor MCP-1. This study provides preclinical evidence in a spontaneous model of breast cancer that mammary adipose tissue inflammation induced by diet, enhances the recruitment of macrophages and increases tumor vascular density suggesting a role for obesity in creating a microenvironment favorable for angiogenesis in the progression of breast cancer.
Keywords: angiogenesis; high-fat diet; inflammation; obesity; tumor progression.
Figures

Similar articles
-
Adipose monocyte chemotactic protein-1 deficiency reduces high-fat diet-enhanced mammary tumorigenesis in MMTV-PyMT mice.J Nutr Biochem. 2020 Mar;77:108313. doi: 10.1016/j.jnutbio.2019.108313. Epub 2019 Nov 29. J Nutr Biochem. 2020. PMID: 31837540
-
Time-restricted feeding mitigates high-fat diet-enhanced mammary tumorigenesis in MMTV-PyMT mice.Nutr Res. 2018 Nov;59:72-79. doi: 10.1016/j.nutres.2018.07.014. Epub 2018 Jul 31. Nutr Res. 2018. PMID: 30442235
-
High-fat Diet Enhances Mammary Tumorigenesis and Pulmonary Metastasis and Alters Inflammatory and Angiogenic Profiles in MMTV-PyMT Mice.Anticancer Res. 2016 Dec;36(12):6279-6287. doi: 10.21873/anticanres.11223. Anticancer Res. 2016. PMID: 27919947
-
Obesity and Breast Cancer: The Role of Crown-Like Structures in Breast Adipose Tissue in Tumor Progression, Prognosis, and Therapy.J Breast Cancer. 2020 Jun;23(3):233-245. doi: 10.4048/jbc.2020.23.e35. J Breast Cancer. 2020. PMID: 32595986 Free PMC article. Review.
-
Molecular Mechanisms of Western Diet-Induced Obesity and Obesity-Related Carcinogenesis-A Narrative Review.Metabolites. 2023 May 21;13(5):675. doi: 10.3390/metabo13050675. Metabolites. 2023. PMID: 37233716 Free PMC article. Review.
Cited by
-
Enriched environment inhibits breast cancer progression in obese models with intact leptin signaling.Endocr Relat Cancer. 2019 May;26(5):483-495. doi: 10.1530/ERC-19-0075. Endocr Relat Cancer. 2019. PMID: 30856610 Free PMC article.
-
A Milk-Fat Based Diet Increases Metastasis in the MMTV-PyMT Mouse Model of Breast Cancer.Nutrients. 2021 Jul 15;13(7):2431. doi: 10.3390/nu13072431. Nutrients. 2021. PMID: 34371939 Free PMC article.
-
Targeting the SphK1/S1P/S1PR1 Axis That Links Obesity, Chronic Inflammation, and Breast Cancer Metastasis.Cancer Res. 2018 Apr 1;78(7):1713-1725. doi: 10.1158/0008-5472.CAN-17-1423. Epub 2018 Jan 19. Cancer Res. 2018. PMID: 29351902 Free PMC article.
-
Macrophages induce malignant traits in mammary epithelium via IKKε/TBK1 kinases and the serine biosynthesis pathway.EMBO Mol Med. 2020 Feb 7;12(2):e10491. doi: 10.15252/emmm.201910491. Epub 2020 Jan 13. EMBO Mol Med. 2020. PMID: 31930708 Free PMC article.
-
Role of n-3 Polyunsaturated Fatty Acids and Exercise in Breast Cancer Prevention: Identifying Common Targets.Nutr Metab Insights. 2016 Oct 30;9:71-84. doi: 10.4137/NMI.S39043. eCollection 2016. Nutr Metab Insights. 2016. PMID: 27812288 Free PMC article. Review.
References
-
- Rinaldi S., Key T.J., Peeters P.H., Lahmann P.H., Lukanova A., Dossus L., Biessy C., Vineis P., Sacerdote C., Berrino F., et al. Anthropometric measures, endogenous sex steroids and breast cancer risk in postmenopausal women: a study within the EPIC cohort. Int. J. Cancer. 2006;118:2832–2839. doi: 10.1002/ijc.21730. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

