Nisin ZP, a Bacteriocin and Food Preservative, Inhibits Head and Neck Cancer Tumorigenesis and Prolongs Survival
- PMID: 26132406
- PMCID: PMC4489501
- DOI: 10.1371/journal.pone.0131008
Nisin ZP, a Bacteriocin and Food Preservative, Inhibits Head and Neck Cancer Tumorigenesis and Prolongs Survival
Abstract
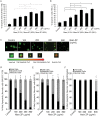
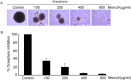
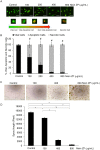
The use of small antimicrobial peptides or bacteriocins, like nisin, to treat cancer is a new approach that holds great promise. Nisin exemplifies this new approach because it has been used safely in humans for many years as a food preservative, and recent laboratory studies support its anti-tumor potential in head and neck cancer. Previously, we showed that nisin (2.5%, low content) has antitumor potential in head and neck squamous cell carcinoma (HNSCC) in vitro and in vivo. The current studies explored a naturally occurring variant of nisin (nisin ZP; 95%, high content) for its antitumor effects in vitro and in vivo. Nisin ZP induced the greatest level of apoptosis in HNSCC cells compared to low content nisin. HNSCC cells treated with increasing concentrations of nisin ZP exhibited increasing levels of apoptosis and decreasing levels of cell proliferation, clonogenic capacity, and sphere formation. Nisin ZP induced apoptosis through a calpain-dependent pathway in HNSCC cells but not in human oral keratinocytes. Nisin ZP also induced apoptosis dose-dependently in human umbilical vein endothelial cells (HUVEC) with concomitant decreases in vascular sprout formation in vitro and reduced intratumoral microvessel density in vivo. Nisin ZP reduced tumorigenesis in vivo and long-term treatment with nisin ZP extended survival. In addition, nisin treated mice exhibited normal organ histology with no evidence of inflammation, fibrosis or necrosis. In summary, nisin ZP exhibits greater antitumor effects than low content nisin, and thus has the potential to serve as a novel therapeutic for HNSCC.
Conflict of interest statement
Figures





Similar articles
-
Nisin, an apoptogenic bacteriocin and food preservative, attenuates HNSCC tumorigenesis via CHAC1.Cancer Med. 2012 Dec;1(3):295-305. doi: 10.1002/cam4.35. Epub 2012 Oct 2. Cancer Med. 2012. PMID: 23342279 Free PMC article.
-
Antitumor effects of IDN5109 on head and neck squamous cell carcinoma.Oncol Rep. 2006 Feb;15(2):329-34. Oncol Rep. 2006. PMID: 16391850
-
A Novel Sirtuin-3 Inhibitor, LC-0296, Inhibits Cell Survival and Proliferation, and Promotes Apoptosis of Head and Neck Cancer Cells.Anticancer Res. 2016 Jan;36(1):49-60. Anticancer Res. 2016. PMID: 26722027 Free PMC article.
-
C-Met pathway promotes self-renewal and tumorigenecity of head and neck squamous cell carcinoma stem-like cell.Oral Oncol. 2014 Jul;50(7):633-9. doi: 10.1016/j.oraloncology.2014.04.004. Epub 2014 May 15. Oral Oncol. 2014. PMID: 24835851 Review.
-
Eating Green: Shining Light on the Use of Dietary Phytochemicals as a Modern Approach in the Prevention and Treatment of Head and Neck Cancers.Curr Top Med Chem. 2018;18(3):182-191. doi: 10.2174/1568026618666180112160713. Curr Top Med Chem. 2018. PMID: 29332583 Review.
Cited by
-
Modulation of pathogenic oral biofilms towards health with nisin probiotic.J Oral Microbiol. 2020 Aug 24;12(1):1809302. doi: 10.1080/20002297.2020.1809302. J Oral Microbiol. 2020. PMID: 32944159 Free PMC article.
-
The Gut Connection: Exploring the Possibility of Implementing Gut Microbial Metabolites in Lymphoma Treatment.Cancers (Basel). 2024 Apr 11;16(8):1464. doi: 10.3390/cancers16081464. Cancers (Basel). 2024. PMID: 38672546 Free PMC article. Review.
-
The Antimicrobial Peptide, Nisin, Synergistically Enhances the Cytotoxic and Apoptotic Effects of Rituximab Treatment on Human Burkitt's Lymphoma Cell Lines.Rep Biochem Mol Biol. 2020 Oct;9(3):250-256. doi: 10.29252/rbmb.9.3.250. Rep Biochem Mol Biol. 2020. PMID: 33649717 Free PMC article.
-
In Vitro Cytotoxic Activity of a Lactococcus lactis Antimicrobial Peptide Against Breast Cancer Cells.Iran J Biotechnol. 2018 Aug 11;16(3):e1867. doi: 10.15171/ijb.1867. eCollection 2018 Aug. Iran J Biotechnol. 2018. PMID: 31457026 Free PMC article.
-
Redefining bioactive small molecules from microbial metabolites as revolutionary anticancer agents.Cancer Gene Ther. 2024 Feb;31(2):187-206. doi: 10.1038/s41417-023-00715-x. Epub 2024 Jan 10. Cancer Gene Ther. 2024. PMID: 38200347 Free PMC article. Review.
References
-
- Nonzee NJ, Dandade NA, Patel U, Markossian T, Agulnik M, Argiris A, et al. Evaluating the supportive care costs of severe radiochemotherapy-induced mucositis and pharyngitis: results from a Northwestern University Costs of Cancer Program pilot study with head and neck and nonsmall cell lung cancer patients who received care at a county hospital, a Veterans Administration hospital, or a comprehensive cancer care center. Cancer. 2008; 113: 1446–1452. 10.1002/cncr.23714 - DOI - PubMed
-
- Bsoul SA, Huber MA, Terezhalmy GT. Squamous cell carcinoma of the oral tissues: a comprehensive review for oral healthcare providers. J Contemp Dent Pract. 2005; 6: 1–16. - PubMed
-
- Farkas-Himsley H, Zhang YS, Yuan M, Musclow CE. Partially purified bacteriocin kills malignant cells by apoptosis: programmed cell death. Cell Mol Biol (Noisy-le-grand). 1992; 38: 643–651. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

