AMPK Phosphorylation Modulates Pain by Activation of NLRP3 Inflammasome
- PMID: 26132721
- PMCID: PMC4742979
- DOI: 10.1089/ars.2014.6120
AMPK Phosphorylation Modulates Pain by Activation of NLRP3 Inflammasome
Abstract
Aims: Impairment in adenosine monophosphate-activated protein kinase (AMPK) activity and NOD-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome activation are associated with several metabolic and inflammatory diseases. In this study, we investigated the role of AMPK/NLRP3 inflammasome axis in the molecular mechanism underlying pain perception.
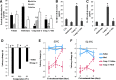
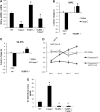
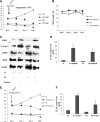
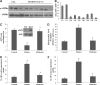
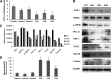
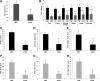
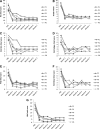
Results: Impairment in AMPK activation induced by compound C or sunitinib, two AMPK inhibitors, provoked hyperalgesia in mice (p<0.001) associated with marked NLRP3 inflammasome protein activation and increased serum levels of interleukin-1β (IL-1β) (24.56±0.82 pg/ml) and IL-18 (23.83±1.882 pg/ml) compared with vehicle groups (IL-1β: 8.15±0.44; IL-18: 4.92±0.4). This effect was rescued by increasing AMPK phosphorylation via metformin treatment (p<0.001), caloric restriction diet (p<0.001), or NLRP3 inflammasome genetic inactivation using NLRP3 knockout (nlrp3(-/-)) mice (p<0.001). Deficient AMPK activation and overactivation of NLRP3 inflammasome axis were also observed in blood cells from patients with fibromyalgia (FM), a prevalent human chronic pain disease. In addition, metformin treatment (200 mg/daily), which increased AMPK activation, restored all biochemical alterations examined by us in blood cells and significantly improved clinical symptoms, such as, pain, fatigue, depression, disturbed sleep, and tender points, in patients with FM.
Innovation and conclusions: These data suggest that AMPK/NLRP3 inflammasome axis participates in chronic pain and that NLRP3 inflammasome inhibition by AMPK modulation may be a novel therapeutic target to fight against chronic pain and inflammatory diseases as FM.
Figures


References
-
- Bagis S, Tamer L, Sahin G, et al. . Free radicals and antioxidants in primary fibromyalgia: an oxidative stress disorder? Rheumatol Int 25: 188–190, 2005 - PubMed
-
- Baroja-Mazo A, Martín-Sánchez F, Gomez AI, Martínez CM, Amores-Iniesta J, Compan V, Barberà-Cremades M, Yagüe J, Ruiz-Ortiz E, Antón J, Buján S, Couillin I, Brough D, Arostegui JI, and Pelegrín P. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol 15: 738–748, 2014 - PubMed
-
- Bazzichi L, Giannaccini G, Betti L, Fabbrini L, Schmid L, Palego L, Giacomelli C, Rossi A, Giusti L, De Feo F, Giuliano T, Mascia G, Bombardieri S, and Lucacchini A. ATP, calcium and magnesium levels in platelets of patients with primary fibromyalgia. Clin Biochem 41: 1084–1090, 2008 - PubMed
-
- Colombo SL. and Moncada S. AMPKalpha1 regulates the antioxidant status of vascular endothelial cells. Biochem J 421: 163–169, 2009 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

