KRAS-dependent sorting of miRNA to exosomes
- PMID: 26132860
- PMCID: PMC4510696
- DOI: 10.7554/eLife.07197
KRAS-dependent sorting of miRNA to exosomes
Abstract
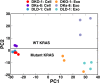
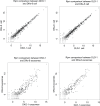
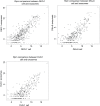
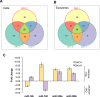
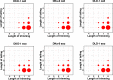
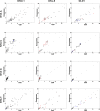
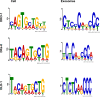
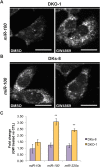
Mutant KRAS colorectal cancer (CRC) cells release protein-laden exosomes that can alter the tumor microenvironment. To test whether exosomal RNAs also contribute to changes in gene expression in recipient cells, and whether mutant KRAS might regulate the composition of secreted microRNAs (miRNAs), we compared small RNAs of cells and matched exosomes from isogenic CRC cell lines differing only in KRAS status. We show that exosomal profiles are distinct from cellular profiles, and mutant exosomes cluster separately from wild-type KRAS exosomes. miR-10b was selectively increased in wild-type exosomes, while miR-100 was increased in mutant exosomes. Neutral sphingomyelinase inhibition caused accumulation of miR-100 only in mutant cells, suggesting KRAS-dependent miRNA export. In Transwell co-culture experiments, mutant donor cells conferred miR-100-mediated target repression in wild-type-recipient cells. These findings suggest that extracellular miRNAs can function in target cells and uncover a potential new mode of action for mutant KRAS in CRC.
Keywords: KRAS; cancer; colorectal cancer; extracellular RNA; human; human biology; medicine; miRNA.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures





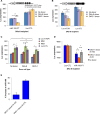

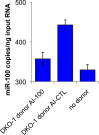
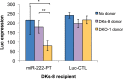
References
-
- Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proceedings of the National Academy of Sciences of USA. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

