H-ficolin binds Aspergillus fumigatus leading to activation of the lectin complement pathway and modulation of lung epithelial immune responses
- PMID: 26133042
- PMCID: PMC4582969
- DOI: 10.1111/imm.12501
H-ficolin binds Aspergillus fumigatus leading to activation of the lectin complement pathway and modulation of lung epithelial immune responses
Abstract
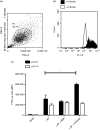
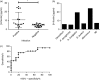
Aspergillus fumigatus is an opportunistic fungal pathogen that typically infects the lungs of immunocompromised patients leading to a high mortality. H-Ficolin, an innate immune opsonin, is produced by type II alveolar epithelial cells and could participate in lung defences against infections. Here, we used the human type II alveolar epithelial cell line, A549, to determine the involvement of H-ficolin in fungal defence. Additionally, we investigated the presence of H-ficolin in bronchoalveolar lavage fluid from transplant patients during pneumonia. H-Ficolin exhibited demonstrable binding to A. fumigatus conidia via l-fucose, d-mannose and N-acetylglucosamine residues in a calcium- and pH-dependent manner. Moreover, recognition led to lectin complement pathway activation and enhanced fungal association with A549 cells. Following recognition, H-ficolin opsonization manifested an increase in interleukin-8 production from A549 cells, which involved activation of the intracellular signalling pathways mitogen-activated protein kinase MAPK kinase 1/2, p38 MAPK and c-Jun N-terminal kinase. Finally, H-ficolin concentrations were significantly higher in bronchoalveolar lavage fluid of patients with lung infections compared with control subjects (n = 16; P = 0·00726). Receiver operating characteristics curve analysis further highlighted the potential of H-ficolin as a diagnostic marker for lung infection (area under the curve = 0·77; P < 0·0001). Hence, H-ficolin participates in A. fumigatus defence through the activation of the lectin complement pathway, enhanced fungus-host interactions and modulated immune responses.
Keywords: Aspergillus; complement; ficolin; innate immunity.
© 2015 John Wiley & Sons Ltd.
Figures



Similar articles
-
The serum opsonin L-ficolin is detected in lungs of human transplant recipients following fungal infections and modulates inflammation and killing of Aspergillus fumigatus.J Infect Dis. 2015 Jul 15;212(2):234-46. doi: 10.1093/infdis/jiv027. Epub 2015 Jan 22. J Infect Dis. 2015. PMID: 25612732
-
Role of ficolin-A and lectin complement pathway in the innate defense against pathogenic Aspergillus species.Infect Immun. 2013 May;81(5):1730-40. doi: 10.1128/IAI.00032-13. Epub 2013 Mar 11. Infect Immun. 2013. PMID: 23478320 Free PMC article.
-
Ficolins Promote Fungal Clearance in vivo and Modulate the Inflammatory Cytokine Response in Host Defense against Aspergillus fumigatus.J Innate Immun. 2016;8(6):579-588. doi: 10.1159/000447714. Epub 2016 Jul 29. J Innate Immun. 2016. PMID: 27467404 Free PMC article.
-
Interference of Aspergillus fumigatus with the immune response.Semin Immunopathol. 2015 Mar;37(2):141-52. doi: 10.1007/s00281-014-0465-1. Epub 2014 Nov 18. Semin Immunopathol. 2015. PMID: 25404120 Free PMC article. Review.
-
Bronchial Epithelial Cells on the Front Line to Fight Lung Infection-Causing Aspergillus fumigatus.Front Immunol. 2020 May 22;11:1041. doi: 10.3389/fimmu.2020.01041. eCollection 2020. Front Immunol. 2020. PMID: 32528481 Free PMC article. Review.
Cited by
-
Differential Interactions of Serum and Bronchoalveolar Lavage Fluid Complement Proteins with Conidia of Airborne Fungal Pathogen Aspergillus fumigatus.Infect Immun. 2020 Aug 19;88(9):e00212-20. doi: 10.1128/IAI.00212-20. Print 2020 Aug 19. Infect Immun. 2020. PMID: 32571987 Free PMC article.
-
Anti-Aspergillus Activities of the Respiratory Epithelium in Health and Disease.J Fungi (Basel). 2018 Jan 8;4(1):8. doi: 10.3390/jof4010008. J Fungi (Basel). 2018. PMID: 29371501 Free PMC article. Review.
-
Humoral Immunity Against Aspergillus fumigatus.Mycopathologia. 2023 Oct;188(5):603-621. doi: 10.1007/s11046-023-00742-0. Epub 2023 Jun 8. Mycopathologia. 2023. PMID: 37289362 Free PMC article. Review.
-
M-ficolin is present in Aspergillus fumigatus infected lung and modulates epithelial cell immune responses elicited by fungal cell wall polysaccharides.Virulence. 2017 Nov 17;8(8):1870-1879. doi: 10.1080/21505594.2016.1278337. Epub 2017 Feb 16. Virulence. 2017. PMID: 28060571 Free PMC article. No abstract available.
-
Ficolins and the Recognition of Pathogenic Microorganisms: An Overview of the Innate Immune Response and Contribution of Single Nucleotide Polymorphisms.J Immunol Res. 2019 Feb 5;2019:3205072. doi: 10.1155/2019/3205072. eCollection 2019. J Immunol Res. 2019. PMID: 30868077 Free PMC article. Review.
References
-
- Steinbach WJ, Marr KA, Anaissie EJ, Azie N, Quan SP, Meier-Kriesche HU, Apewokin S, Horn DL. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J Infect. 2012;65:453–64. - PubMed
-
- NHS. [WWW document]. URL http://www.nhs.uk/conditions/Aspergillosis/Pages/Introduction.aspx. [accessed on 25 May 2015]
-
- Schelenz S, Goldsmith DJ. Aspergillus endophthalmitis: an unusual complication of disseminated infection in renal transplant patients. J Infect. 2003;47:336–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

