Interventions for post-stroke fatigue
- PMID: 26133313
- PMCID: PMC7387276
- DOI: 10.1002/14651858.CD007030.pub3
Interventions for post-stroke fatigue
Abstract
Background: Post-stroke fatigue (PSF) is a common and distressing problem after stroke. The best ways to prevent or treat PSF are uncertain. Several different interventions can be argued to have a rational basis.
Objectives: To determine whether, among people with stroke, any intervention reduces the proportion of people with fatigue, fatigue severity, or both; and to determine the effect of intervention on health-related quality of life, disability, dependency and death, and whether such intervention is cost effective.
Search methods: We searched the Cochrane Stroke Group Trials Register (last searched May 2014), Cochrane Central Register of Controlled Trials (The Cochrane Library, 2014, Issue 4), MEDLINE (1950 to May 2014), EMBASE (1980 to May 2014), CINAHL (1982 to May 2014), AMED (1985 to May 2014), PsycINFO (1967 to May 2014), Digital Dissertations (1861 to May 2014), British Nursing Index (1985 to May 2014), PEDro (searched May 2014) and PsycBITE (searched May 2014). We also searched four ongoing trials registries, scanned reference lists, performed citation tracking of included trials and contacted experts.
Selection criteria: Two review authors independently scrutinised all titles and abstracts and excluded obviously irrelevant studies. We obtained the full texts for potentially relevant studies and three review authors independently applied the inclusion criteria. We included randomised controlled trials (RCTs) that compared an intervention with a control, or compared different interventions for PSF.
Data collection and analysis: Two review authors independently extracted data and assessed risk of bias for each included trial. The primary outcomes were severity of fatigue, or proportion of people with fatigue after treatment. We performed separate analyses for trials investigating efficacy in treating PSF, trials investigating efficacy in preventing PSF and trials not primarily investigating efficacy in PSF but which reported fatigue as an outcome. We pooled results from trials that had a control arm. For trials that compared different potentially active interventions without a control arm, we performed analyses for individual trials without pooling.We calculated standardised mean difference (SMD) as the effect size for continuous outcomes and risk ratio (RR) for dichotomous outcomes. We pooled the results using a random-effects model and assessed heterogeneity using the I(2) statistic. We performed separate subgroup analyses for pharmacological and non-pharmacological interventions. We also performed sensitivity analyses to assess the influence of methodological quality.
Main results: We retrieved 12,490 citations, obtained full texts for 58 studies and included 12 trials (three from the 2008 search and nine from the 2014 search) with 703 participants. Eight trials primarily investigated the efficacy in treating PSF, of which six trials with seven comparisons provided data suitable for meta-analysis (five pharmacological interventions: fluoxetine, enerion, (-)-OSU6162, citicoline and a combination of Chinese herbs; and two non-pharmacological interventions: a fatigue education programme and a mindfulness-based stress reduction programme). The fatigue severity was lower in the intervention groups than in the control groups (244 participants, pooled SMD -1.07, 95% confidence interval (CI) -1.93 to -0.21), with significant heterogeneity between trials (I(2) = 87%, degrees of freedom (df) = 6, P value < 0.00001). The beneficial effect was not seen in trials that had used adequate allocation concealment (two trials, 89 participants, SMD -0.38, 95% CI -0.80 to 0.04) or trials that had used adequate blinding of outcome assessors (four trials, 198 participants, SMD -1.10, 95% CI -2.31 to 0.11).No trial primarily investigated the efficacy in preventing PSF.Four trials (248 participants) did not primarily investigate the efficacy on fatigue but other symptoms after stroke. None of these interventions showed any benefit on reducing PSF, which included tirilazad mesylate, continuous positive airway pressure for sleep apnoea, antidepressants and a self management programme for recovery from chronic diseases.
Authors' conclusions: There was insufficient evidence on the efficacy of any intervention to treat or prevent fatigue after stroke. Trials to date have been small and heterogeneous, and some have had a high risk of bias. Some of the interventions described were feasible in people with stroke, but their efficacy should be investigated in RCTs with a more robust study design and adequate sample sizes.
Conflict of interest statement
Simiao Wu: none known. Mansur A Kutlubaev: none known. Ho‐Yan Y Chun: none known. Eileen Cowey: none known. Alex Pollock: none known. Malcolm Macleod: is an employee of the University of Edinburgh and the Medicines & Healthcare products Regulatory Agency (MHRA), and receives honoraria relating to book and journal publishing. Martin Dennis: none known. Elizabeth Keane: none known. Michael Sharpe: for the 2008 review, Michael Sharpe received a research grant from the Scottish Government Chief Scientist Office to carry out research on a related topic. He is currently employed by the University of Oxford. He has no competing interests. Gillian Mead: has been awarded a project grant from the Scottish Government Chief Scientist Office to perform a longitudinal study of fatigue after stroke. The preliminary results of this Cochrane review were used in the application for funding to justify the need for further studies in this area. She has developed a course on exercise after stroke, which was licensed to Later Life Training who pay royalties for the course. She has received expenses for speaking at conferences on exercise and fatigue after stroke.
Figures

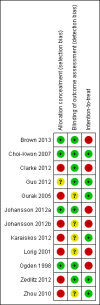










Update of
-
Interventions for post-stroke fatigue.Cochrane Database Syst Rev. 2009 Jul 8;(3):CD007030. doi: 10.1002/14651858.CD007030.pub2. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2015 Jul 02;(7):CD007030. doi: 10.1002/14651858.CD007030.pub3. PMID: 19588416 Updated.
References
References to studies included in this review
Brown 2013 {published data only}
Choi‐Kwon 2007 {published data only}
-
- Choi‐Kwon S, Choi J, Kang D‐W, Kim JS. Fluoxetine is not effective in the treatment of post‐stroke fatigue: a double‐blind, placebo‐controlled study. Cerebrovascular Diseases 2007;23:102‐8. - PubMed
Clarke 2012 {published data only}
-
- Clarke A, Barker‐Collo S, Feigin V. Poststroke fatigue: does group education make a difference? A randomized pilot trial. Topics in Stroke Rehabilitation 2012;19:32‐9. - PubMed
Guo 2012 {published data only}
-
- Guo YH, Chen HX, Xie RM. Effects of qi‐supplementing dominated Chinese materia medica combined with rehabilitation training on the quality of life of ischemic post‐stroke fatigue patients of qi deficiency syndrome [Chinese]. Zhongguo Zhong xi yi jie he za zhi [Chinese Journal of Integrated Traditional and Western Medicine] 2012;32:160‐3. - PubMed
Gurak 2005 {published data only}
-
- Gurak SV, Parfenov VA. Asthenia after stroke and myocardial infarction and its treatment with Enerion. Klinicheskaya Gerontologia 2005;8:9‐12.
Johansson 2012a {published data only}
-
- Johansson B, Carlsson A, Carlsson ML, Karlsson M, Nilsson MKL, Nordquist‐Brandt E. Placebo‐controlled cross‐over study of the monoaminergic stabiliser (‐)‐OSU6162 in mental fatigue following stroke or traumatic brain injury. Acta Neuropsychiatrica 2012;24:266‐74. - PubMed
Johansson 2012b {published data only}
-
- Johansson B, Bjuhr H, Rönnbäck L. Mindfulness‐based stress reduction (MBSR) improves long‐term mental fatigue after stroke or traumatic brain injury. Brain Injury 2012;26:1621‐8. - PubMed
Karaiskos 2012 {published data only}
-
- Karaiskos D, Tzavellas E, Spengos K, Vassilopoulou S, Paparrigopolous T. Duloxetine versus citalopram and sertraline in the treatment of poststroke depression, anxiety, and fatigue. Journal of Neuropsychiatry and Clinical Neurosciences 2012;24:349‐53. - PubMed
Lorig 2001 {published data only}
-
- Lorig KR, Ritter P, Stewart AL, Sobel DS, Brown BW, Bandura A, et al. Chronic disease self‐management program: 2‐year health status and health care utilization outcomes. Medical Care 2001;39:1217–23. - PubMed
Ogden 1998 {published data only}
-
- Ogden JA, Mee EW, Utley T. Too little, too late: does tirilazad mesylate reduce fatigue after subarachnoid haemorrhage?. Neurosurgery 1998;4:782‐7. - PubMed
Zedlitz 2012 {published data only}
-
- Zedlitz AMEE, Rietveld TCM, Geurts AC, Fasotti L. Cognitive and graded activity training can alleviate persistent fatigue after stroke: a randomized, controlled trial. Stroke 2012;43:1046‐51. - PubMed
Zhou 2010 {published data only}
-
- Zhou Y, Zhou GY, Li SK, Jin JH. Clinical observation on the therapeutic effect of electroacupuncture combined with cupping on post‐stroke fatigue [Chinese]. Zhen Ci Yan Jiu 2010;35:380‐3. - PubMed
References to studies excluded from this review
Allison 2007 {published data only}
-
- Allison R, Dennett R. Pilot randomized controlled trial to assess the impact of additional supported standing practice on functional ability post stroke. Clinical Rehabilitation 2007;21:614‐9. - PubMed
Brioschi 2009 {published data only}
-
- Brioschi A, Gramigna S, Werth E, Staub F, Ruffieux C, Bassetti C, et al. Effect of modafinil on subjective fatigue in multiple sclerosis and stroke patients. European Neurology 2009;62:243‐9. - PubMed
Cruz 2013 {published data only}
-
- Cruz TV. Stroke wearable operative rehabilitation device impact trial (SWORD‐IT). clinicaltrials.gov/show/NCT01967290 (accessed 21 June 2015).
Feys 2013 {published data only}
-
- Feys P. Effect of I‐TRAVLE training on arm function in MS and chronic stroke patients. clinicaltrials.gov/show/NCT01918748 (accessed 21 June 2015).
Kim 2012 {published data only}
-
- Kim I. Effects of an enjoyable nurse‐led intervention to promote movement in poststroke inpatients. Clinical Nursing Research 2012;21:390‐405. - PubMed
Kirkevold 2012 {published data only}
-
- Kirkevold M. Poststroke fatigue ‐ developing and testing a program to reduce cope with fatigue. clinicaltrials.gov/show/NCT01629654 (accessed 21 June 2015).
Lin 2013 {published data only}
-
- Lin KC. Comparative efficacy research of robot‐assisted therapy with and without constraint‐induced therapy in stroke rehabilitation. clinicaltrials.gov/show/NCT01907139 (accessed 21 June 2015).
Robinson 2003 {published data only}
-
- Robinson S, Vollmer C, Hermes B. A program to reduce fatigue in convalescing elderly adults. Journal of Gerontological Nursing 2003;29:47‐53. - PubMed
Sianni 2008 {published data only}
-
- Sianni A. The benefiting effect of exercise after an acute cerebrovascular stroke (ACVS). International Journal of Stroke 2008;3 Suppl 1:468.
Underwood 2006 {published data only}
-
- Underwood J, Clark PC, Blanton S, Aycock DM, Wolf SL. Pain, fatigue, and intensity of practice in people with stroke who are receiving constraint‐induced movement therapy. Physical Therapy 2006;86:1241‐50. - PubMed
Wu 2014b {published data only}
-
- Wu S. A rehabilitation therapy for post‐stroke fatigue. clinicaltrials.gov/show/NCT02131532 (accessed 21 June 2015).
References to ongoing studies
AFFINITY 2013 {published data only}
-
- Hackett M, Hankey G. Assessment of fluoxetine in stroke recovery (AFFINITY) trial. www.affinitytrial.org/download/study_outline/Protocol%20Summary%20V3.pdf (accessed 21 June 2015).
Chuang 2013 {published data only}
-
- Chuang L‐L. A study of post‐stroke pain and fatigue: clinical evaluation and treatment effect. clinicaltrials.gov/show/NCT01913509 (accessed 21 June 2015).
EFFECTS 2014 {published data only}
-
- Laska AC, Lundström E, Berthold E, Markaki I, Löfmark U, Wiberg B, et al. Efficacy oF Fluoxetine ‐ a randomisEd Controlled Trials in Stroke. Determination of the efficacy and safety of fluoxetine treatment for stroke ‐ a randomized placebo‐controlled study of 1500 patients. www.effects.se/?page_id=114 (accessed 21 June 2015).
FOCUS 2012 {published data only}
-
- Mead GE. Fluoxetine Or Control Under Supervision (FOCUS) trial. www.focustrial.org.uk/default.html (accessed 21 June 2015).
Liu 2012 {published data only}
-
- Liu C‐H. Randomized, double blind, placebo control trial to evaluate the efficacy of Astragalus membranaceus in the patients after stroke with fatigue. clinicaltrials.gov/ct2/show/NCT01554787 (accessed 21 June 2015).
MacKay‐Lyons 2012 {published data only}
-
- MacKay‐Lyons M. Combined effects of aerobic exercise and cognitive training on cognition after stroke. clinicaltrials.gov/ct2/show/NCT01674790 (accessed 21 June 2015).
Michael 2008 {published data only}
-
- Michael KM. Testing Adaptive Physical Activity in Stroke (TAPAS). clinicaltrials.gov/ct2/show/NCT01042990 (accessed 21 June 2015).
Overgaard 2012 {published data only}
-
- Overgaard K. Poulsen M. Treatment of post stroke fatigue with a wakefulness promoting agent. clinicaltrials.gov/ct2/show/NCT01800097 (accessed 21 June 2015).
Vanroy 2010 {published data only}
-
- Vanroy C, Cras P, Vanlandewijck Y. The effect of an aerobic exercise programme in stroke patients. clinicaltrials.gov/ct2/show/NCT01070459 (accessed 21 June 2015).
Additional references
Armes 2007
-
- Armes J, Chalder T, Addington‐Hall J, Richardson A, Hotopf M. A randomized controlled trial to evaluate the effectiveness of a brief, behaviorally oriented intervention for cancer‐related fatigue. Cancer 2007;110:1385‐95. - PubMed
Borenstein 2009
-
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Subgroup analyses. Introduction to Meta‐Analysis. 1st Edition. Chichester, West Sussex, UK: John Wiley & Sons, 2009:149‐86.
Boĭko 2013
-
- Boĭko AN, Lebedeva AV, Shchukin IA, Soldatov MA, Petrov SV, Khozova AA, et al. Emotional disorders and quality of life in patients with post stroke asthenia. Zhurnal Nevrologii i Psikhiatrii Imeni S.S. Korsakova 2013;113(11):27‐33. - PubMed
Choi‐Kwon 2011
-
- Choi‐Kwon S, Kim JS. Poststroke fatigue: an emerging, critical issue in stroke medicine. International Journal of Stroke 2011;6:328‐36. - PubMed
Costantini 2014
-
- Costantini A, Pala MI, Catalano ML, Notarangelo C, Careddu P. High‐dose thiamine improves fatigue after stroke: a report of three cases. Journal of Alternative and Complementary Medicine 2014;20:683‐5. - PubMed
Deeks 2001
-
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey‐Smith G, Altman DG editor(s). Systematic Reviews in Healthcare: Meta‐analysis in Context. 2nd Edition. London: BMJ Publishing Group, 2001.
Duncan 2015
-
- Duncan F, Lewis SJ, Greig CA, Dennis MS, Sharpe M, MacLullich AM, et al. Exploratory longitudinal cohort study of associations of fatigue after stroke. Stroke 2015;46:1052‐8. - PubMed
Glader 2002
-
- Glader E‐L, Stegmayr B, Asplund K. Post‐stroke fatigue. A 2‐year follow‐up study of stroke patients in Sweden. Stroke 2002;33:1327‐33. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011. Available from www.cochrane‐handbook.org.
Huijts 2012
Kalinskiĭ 2008
-
- Kalinskiĭ PP, Nazarov VV, Samarets NA, Ulitina MN. Peculiarities of treatment of asthenic syndrome in the acute period of ischemic stroke. Zhurnal Nevrologii i Psikhiatrii Imeni S.S. Korsakova 2008;108:72‐4. - PubMed
Krupp 1989
-
- Krupp LB, LaRocca NG, Muir‐Nash J, Steinberg AD. The Fatigue Severity Scale. Archives of Neurology 1989;46:1121‐3. - PubMed
Kuppuswamy 2015
Kutlubaev 2012
-
- Kutlubaev MA, Duncan FH, Mead GE. Biological correlates of post‐stroke fatigue: a systematic review. Acta Neurologica Scandinavica 2012;125:219‐27. - PubMed
Lewis 2011
-
- Lewis SJ, Barugh AJ, Greig CA, Saunders DH, Fitzsimons C, Dinan‐Young S, et al. Is fatigue after stroke associated with physical deconditioning? A cross‐sectional study in ambulatory stroke survivors. Archives of Physical Medicine and Rehabilitation 2011;92:295‐8. - PubMed
Lynch 2007
-
- Lynch J, Mead G, Greig C, Young A, Lewis S, Sharpe M. Fatigue after stroke: the development and evaluation of a case definition. Journal of Psychosomatic Research 2007;63:539‐44. - PubMed
Mead 2007
-
- Mead G, Lynch J, Greig C, Young A, Lewis S, Sharpe M. Evaluation of fatigue scales in stroke patients. Stroke 2007;38:2090‐5. - PubMed
Michael 2006
-
- Michael KM, Allen JK, Macko RF. Fatigue after stroke: relationship to mobility, fitness, ambulatory activity, social support and falls efficacy. Rehabilitation Nursing 2006;5:210‐7. - PubMed
Ormstad 2011
Ormstad 2014
-
- Ormstad H, Verkerk R, Amthor K‐F, Sandvik L. Activation of the kynurenine pathway in the acute phase of stroke and its role in fatigue and depression following stroke. Journal of Molecular Neuroscience 2014;54:1‐7. - PubMed
Passier 2011
-
- Passier PE, Post MW, Zandvoort MJ, Rinkel GJ, Lindeman E, Visser‐Meily JM. Predicting fatigue 1 year after aneurysmal subarachnoid hemorrhage. Journal of Neurology 2011;258:1091‐7. - PubMed
Payne 2012
Pollock 2012
-
- Pollock A, George B, Fenton M, Firkins L. Top ten research priorities relating to life after stroke. Lancet Neurology 2012;11:209. - PubMed
Radman 2012
-
- Radman N, Staub F, Aboulafia‐Brakha T, Berney A, Bogousslavsky J, Annoni JM. Poststroke fatigue following minor infarcts: a prospective study. Neurology 2012;79:1422‐7. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2014.
Saunders 2013
Syed 2007
-
- Syed AB, Castell LM, Ng A, Winward C, Rothwell PM. Plasma glutamate levels predict fatigue after TIA and minor stroke. Cerebrovascular Diseases 2007;23 Suppl 2:117.
Tyson 2014
-
- Tyson SF, Brown P. How to measure fatigue in neurological conditions? A systematic review of psychometric properties and clinical utility of measures used so far. Clinical Rehabilitation 2014;28:804‐16. - PubMed
van de Port 2007
-
- Port IG, Kwakkel G, Schepers VP, Heinemans CT, Lindeman E. Is fatigue an independent factor associated with activities of daily living, instrumental activities of daily living and health‐related quality of life in chronic stroke?. Cerebrovascular Diseases 2007;23:40‐5. - PubMed
White 2011
Whiting 2001
Wu 2014a
Wu 2015
-
- Wu S, Mead G, Macleod M, Chalder T. A model of understanding fatigue after stroke. Stroke 2015;46:893‐8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

