Mutations in GMPPB cause congenital myasthenic syndrome and bridge myasthenic disorders with dystroglycanopathies
- PMID: 26133662
- PMCID: PMC4547052
- DOI: 10.1093/brain/awv185
Mutations in GMPPB cause congenital myasthenic syndrome and bridge myasthenic disorders with dystroglycanopathies
Abstract
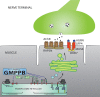
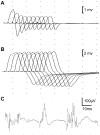
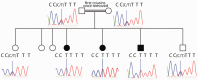
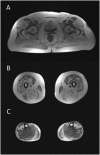
Congenital myasthenic syndromes are inherited disorders that arise from impaired signal transmission at the neuromuscular junction. Mutations in at least 20 genes are known to lead to the onset of these conditions. Four of these, ALG2, ALG14, DPAGT1 and GFPT1, are involved in glycosylation. Here we identify a fifth glycosylation gene, GMPPB, where mutations cause congenital myasthenic syndrome. First, we identified recessive mutations in seven cases from five kinships defined as congenital myasthenic syndrome using decrement of compound muscle action potentials on repetitive nerve stimulation on electromyography. The mutations were present through the length of the GMPPB, and segregation, in silico analysis, exon trapping, cell transfection followed by western blots and immunostaining were used to determine pathogenicity. GMPPB congenital myasthenic syndrome cases show clinical features characteristic of congenital myasthenic syndrome subtypes that are due to defective glycosylation, with variable weakness of proximal limb muscle groups while facial and eye muscles are largely spared. However, patients with GMPPB congenital myasthenic syndrome had more prominent myopathic features that were detectable on muscle biopsies, electromyography, muscle magnetic resonance imaging, and through elevated serum creatine kinase levels. Mutations in GMPPB have recently been reported to lead to the onset of muscular dystrophy dystroglycanopathy. Analysis of four additional GMPPB-associated muscular dystrophy dystroglycanopathy cases by electromyography found that a defective neuromuscular junction component is not always present. Thus, we find mutations in GMPPB can lead to a wide spectrum of clinical features where deficit in neuromuscular transmission is the major component in a subset of cases. Clinical recognition of GMPPB-associated congenital myasthenic syndrome may be complicated by the presence of myopathic features, but correct diagnosis is important because affected individuals can respond to appropriate treatments.
Keywords: GMPPB; congenital myasthenic syndrome; dystroglycan; glycosylation; neurotransmission defect.
© The Author (2015). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures




References
-
- Chaouch A, Müller J, Lochmüller H. A retrospective clinical study of the treatment of slow-channel congenital myasthenic syndrome. J Neurol 2012; 259: 474. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

