The role and potential of umbilical cord blood in an era of new therapies: a review
- PMID: 26133757
- PMCID: PMC4489204
- DOI: 10.1186/s13287-015-0113-2
The role and potential of umbilical cord blood in an era of new therapies: a review
Abstract
In light of pioneering findings in the 1980s and an estimation of more than 130 million global annual births, umbilical cord blood (UCB) is considered to be the most plentiful reservoir of cells and to have regenerative potential for many clinical applications. Although UCB is used mainly against blood disorders, the spectrum of diseases for which it provides effective therapy has been expanded to include non-hematopoietic conditions; UCB has also been used as source for regenerative cell therapy and immune modulation. Thus, collection and banking of UCB-derived cells have become a popular option. However, there are questions regarding the cost versus the benefits of UCB banking, and it also raises complex ethical and legal issues. This review discusses many issues surrounding the conservation of UCB-derived cells and the great potential and current clinical applications of UCB in an era of new therapies. In particular, we describe the practical issues inherent in UCB collection, processing, and long-term storage as well as the different types of 'stem' or progenitor cells circulating in UCB and their uses in multiple clinical settings. Given these considerations, the trend toward UCB will continue to provide growing assistance to health care worldwide.
Figures
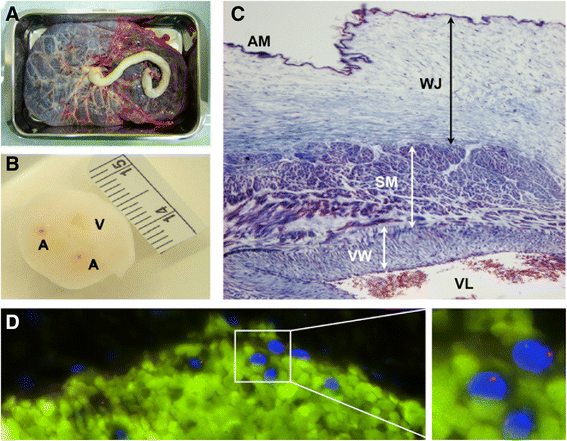
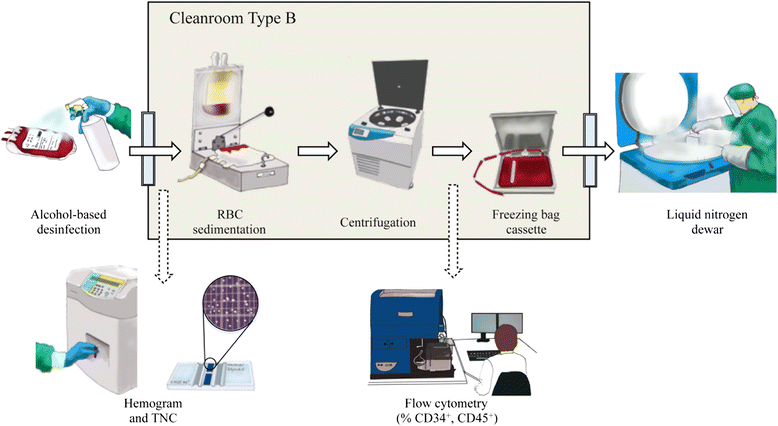
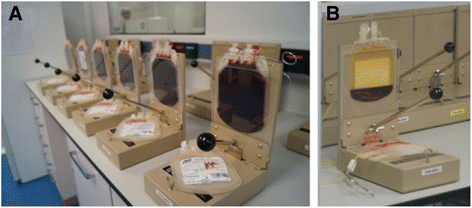

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

