A Highlight of Recent Advances in Aptamer Technology and Its Application
- PMID: 26133761
- PMCID: PMC6331864
- DOI: 10.3390/molecules200711959
A Highlight of Recent Advances in Aptamer Technology and Its Application
Abstract
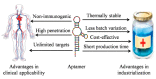
Aptamers and SELEX (systematic evolution of ligands by exponential enrichment) technology have gained increasing attention over the past 25 years. Despite their functional similarity to protein antibodies, oligonucleotide aptamers have many unique properties that are suitable for clinical applications and industrialization. Aptamers may be superior to antibodies in fields such as biomarker discovery, in vitro and in vivo diagnosis, precisely controlled drug release, and targeted therapy. However, aptamer commercialization has not occurred as quickly as expected, and few aptamer-based products have yet successfully entered clinical and industrial use. Thus, it is important to critically review some technical barriers of aptamer and SELEX technology per se that may impede aptamer development and application. To date, how to rapidly obtain aptamers with superior bioavailability over antibodies remains the key issue. In this review, we discuss different chemical and structural modification strategies aimed to enhance aptamer bioavailability. We also discuss improvements to SELEX process steps to shorten the selection period and improve the SELEX process success rate. Applications in which aptamers are particularly suited and perform differently or superior to antibodies are briefly introduced.
Keywords: SELEX; application; aptamer; improvement; modification; optimization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

