Induction of Mitochondrial Dependent Apoptosis in Human Leukemia K562 Cells by Meconopsis integrifolia: A Species from Traditional Tibetan Medicine
- PMID: 26133762
- PMCID: PMC6332253
- DOI: 10.3390/molecules200711981
Induction of Mitochondrial Dependent Apoptosis in Human Leukemia K562 Cells by Meconopsis integrifolia: A Species from Traditional Tibetan Medicine
Abstract
Objectives: Meconopsis integrifolia (M. integrifolia) is one of the most popular members in Traditional Tibetan Medicine. This study aimed to investigate the anticancer effect of M. integrifolia and to detect the underlying mechanisms of these effects.
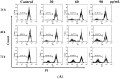
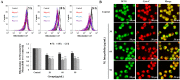
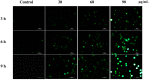
Methods: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay and trypan blue assay were used to evaluate the cytotoxicity of M. integrifolia. Changes in cell nuclear morphology and reactive oxygen species (ROS) level were observed by fluorescent microscopy. Apoptosis ratio, DNA damage and mitochondrial membrane potential (MMP) loss were analyzed by flow cytometry. Western blotting assay was adopted to detect the proteins related to apoptosis. Immunofluorescence was used to observe the release of cytochrome C.
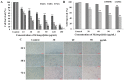
Results: The obtained data revealed that M. integrifolia could significantly inhibit K562 cell viability, mainly by targeting apoptosis induction and cell cycle arrest in G2/M phase. Collapse in cell morphology, chromatin condensation, DNA damage and ROS accumulation were observed. Further mechanism detection revealed that mitochondrion might be a key factor in M. integrifolia-induced apoptosis.
Conclusions: M. integrifolia could induce mitochondria mediated apoptosis and cell cycle arrest in G2/M phase with little damage to normal cells, suggesting that M. integrifolia might be a potential and efficient anticancer agent that deserves further investigation.
Keywords: G2/M phase arrest; K562 cells; M. integrifolia ethanol extract; apoptosis; mitochondria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Cucurbitacin B induces DNA damage, G2/M phase arrest, and apoptosis mediated by reactive oxygen species (ROS) in leukemia K562 cells.Anticancer Agents Med Chem. 2014;14(8):1146-53. doi: 10.2174/1871520614666140601220915. Anticancer Agents Med Chem. 2014. PMID: 24893803
-
The pharmacological NFkappaB inhibitors BAY117082 and MG132 induce cell arrest and apoptosis in leukemia cells through ROS-mitochondria pathway activation.Cancer Lett. 2010 Feb 28;288(2):192-203. doi: 10.1016/j.canlet.2009.06.038. Epub 2009 Jul 30. Cancer Lett. 2010. PMID: 19646807
-
Benzyl isothiocyanate (BITC) induces G2/M phase arrest and apoptosis in human melanoma A375.S2 cells through reactive oxygen species (ROS) and both mitochondria-dependent and death receptor-mediated multiple signaling pathways.J Agric Food Chem. 2012 Jan 18;60(2):665-75. doi: 10.1021/jf204193v. Epub 2012 Jan 6. J Agric Food Chem. 2012. PMID: 22148415
-
Viscum articulatum Burm. f. aqueous extract exerts antiproliferative effect and induces cell cycle arrest and apoptosis in leukemia cells.J Ethnopharmacol. 2018 Jun 12;219:91-102. doi: 10.1016/j.jep.2018.03.005. Epub 2018 Mar 16. J Ethnopharmacol. 2018. PMID: 29555410
-
Polytrichum commune L.ex Hedw ethyl acetate extract-triggered perturbations in intracellular Ca²⁺ homeostasis regulates mitochondrial-dependent apoptosis.J Ethnopharmacol. 2015 Aug 22;172:410-20. doi: 10.1016/j.jep.2015.07.002. Epub 2015 Jul 4. J Ethnopharmacol. 2015. PMID: 26151243
Cited by
-
Phytochemicals from Ajwa dates pulp extract induce apoptosis in human triple-negative breast cancer by inhibiting AKT/mTOR pathway and modulating Bcl-2 family proteins.Sci Rep. 2021 May 14;11(1):10322. doi: 10.1038/s41598-021-89420-z. Sci Rep. 2021. PMID: 33990623 Free PMC article.
-
Traditional Tibetan Medicine in Cancer Therapy by Targeting Apoptosis Pathways.Front Pharmacol. 2020 Jul 7;11:976. doi: 10.3389/fphar.2020.00976. eCollection 2020. Front Pharmacol. 2020. PMID: 32774302 Free PMC article. Review.
-
Dimethyl Sulfoxide Perturbs Cell Cycle Progression and Spindle Organization in Porcine Meiotic Oocytes.PLoS One. 2016 Jun 27;11(6):e0158074. doi: 10.1371/journal.pone.0158074. eCollection 2016. PLoS One. 2016. PMID: 27348312 Free PMC article.
-
Reactive Oxygen Species-Mediated Mitochondrial Dysfunction Triggers Sodium Valproate-Induced Cytotoxicity in Human Colorectal Adenocarcinoma Cells.J Gastrointest Cancer. 2021 Sep;52(3):899-906. doi: 10.1007/s12029-020-00505-w. J Gastrointest Cancer. 2021. PMID: 32880040
-
Cytostatic and Anti-tumor Potential of Ajwa Date Pulp against Human Hepatocellular Carcinoma HepG2 Cells.Sci Rep. 2019 Jan 21;9(1):245. doi: 10.1038/s41598-018-36475-0. Sci Rep. 2019. PMID: 30664656 Free PMC article.
References
-
- Kapoor L.D. Opium Poppy: Botany, Chemistry and Pharmacology. Food Products Press; New York, NY, USA: 1995.
-
- Wu C., Chuang H. A study on the taxonomic system of the genus Meconopsis. Acta Bot. Yunnanica. 1980;2:371–381.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

