Multitrial Evaluation of Progression-Free Survival as a Surrogate End Point for Overall Survival in First-Line Extensive-Stage Small-Cell Lung Cancer
- PMID: 26134227
- PMCID: PMC4493926
- DOI: 10.1097/JTO.0000000000000548
Multitrial Evaluation of Progression-Free Survival as a Surrogate End Point for Overall Survival in First-Line Extensive-Stage Small-Cell Lung Cancer
Abstract
Introduction: We previously reported that progression-free survival (PFS) may be a candidate surrogate end point for overall survival (OS) in first-line extensive-stage small-cell lung cancer (ES-SCLC) using data from three randomized trials (Foster, Cancer 2011). In this validation study (N0424-Alliance), we assessed the patient-level and trial-level surrogacy of PFS using data from seven new first-line phase II/III ES-SCLC trials and across all 10 trials as well (seven new, three previous).
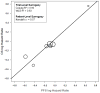
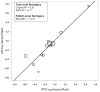
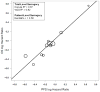
Methods: Individual patient data were utilized across the seven new trials (2259 patients) and all 10 trials (2855 patients). Patient-level surrogacy (Kendall's τ) was assessed using the Clayton copula bivariate survival model. Trial-level surrogacy was assessed through association of the log hazard ratios on OS and PFS across trials, including weighted (by trial size) least squares regression (WLS R²) of Cox model effects and correlation of the copula effects (copula R²). The minimum effect on the surrogate (MES) needed to detect a nonzero treatment effect on OS was also calculated.
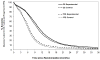
Results: The median OS and PFS across all 10 trials were 9.8 and 5.9 months, respectively. PFS showed strong surrogacy within the 7 new trials (copula R² = 0.90 [standard error = 0.27], WLS R² = 0.83 [95% confidence interval: 0.43, 0.95]; MES = 0.67, and Kendall's τ = 0.58) and across all 10 trials (copula R² = 0.81 [standard errors = 0.25], WLS R² = 0.77 [95% confidence interval: 0.47-0.91], MES = 0.70, and Kendall's τ = 0.57).
Conclusions: PFS demonstrated strong surrogacy for OS in first-line ES-SCLC based on this external validation study of individual patient data. PFS is a good alternative end point to OS and should be considered when resource constraints (time or patient) might make it useful or desirable in place of OS. Additional analyses are needed to assess its appropriateness for targeted agents in this disease setting.
Conflict of interest statement
Conflicts of Interest: For the remaining authors, none were declared.
Figures
References
-
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014 Jan;64(1):9–29. - PubMed
-
- National Cancer Institute. [Accessed in April 2014];US National Institutes of Health website on small-cell lung cancer for health professionals. Available at http://www.cancer.gov/cancertopics/pdq/treatment/small-cell-lung/healthp....
-
- Roth BJ, Johnson DH, Einhorn LH, et al. Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol. 1992;10(2):282–91. - PubMed
Publication types
MeSH terms
Grants and funding
- CA33601/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- U10 CA033601/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- U10 CA066636/CA/NCI NIH HHS/United States
- UG1 CA233180/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- U10 CA180867/CA/NCI NIH HHS/United States
- CA31946/CA/NCI NIH HHS/United States
- CA25224/CA/NCI NIH HHS/United States
- U10 CA023318/CA/NCI NIH HHS/United States
- P01 CA142538/CA/NCI NIH HHS/United States
- U10 CA180794/CA/NCI NIH HHS/United States
- U10 CA025224/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

