Type VI Secretion System Transports Zn2+ to Combat Multiple Stresses and Host Immunity
- PMID: 26134274
- PMCID: PMC4489752
- DOI: 10.1371/journal.ppat.1005020
Type VI Secretion System Transports Zn2+ to Combat Multiple Stresses and Host Immunity
Abstract
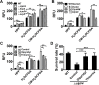
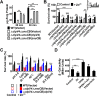
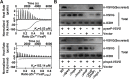
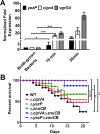
Type VI secretion systems (T6SSs) are widespread multi-component machineries that translocate effectors into either eukaryotic or prokaryotic cells, for virulence or for interbacterial competition. Herein, we report that the T6SS-4 from Yersinia pseudotuberculosis displays an unexpected function in the transportation of Zn2+ to combat diverse stresses and host immunity. Environmental insults such as oxidative stress induce the expression of T6SS-4 via OxyR, the transcriptional factor that also regulates many oxidative response genes. Zinc transportation is achieved by T6SS-4-mediated translocation of a novel Zn2+-binding protein substrate YezP (YPK_3549), which has the capacity to rescue the sensitivity to oxidative stress exhibited by T6SS-4 mutants when added to extracellular milieu. Disruption of the classic zinc transporter ZnuABC together with T6SS-4 or yezP results in mutants that almost completely lost virulence against mice, further highlighting the importance of T6SS-4 in resistance to host immunity. These results assigned an unconventional role to T6SSs, which will lay the foundation for studying novel mechanisms of metal ion uptake by bacteria and the role of this process in their resistance to host immunity and survival in harmful environments.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

