Emergent Properties of the Metaphase Spindle
- PMID: 26134313
- PMCID: PMC4484974
- DOI: 10.1101/cshperspect.a015784
Emergent Properties of the Metaphase Spindle
Abstract
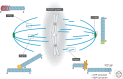
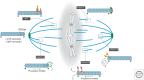
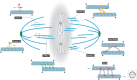
A metaphase spindle is a complex structure consisting of microtubules and a myriad of different proteins that modulate microtubule dynamics together with chromatin and kinetochores. A decade ago, a full description of spindle formation and function seemed a lofty goal. Here, we describe how work in the last 10 years combining cataloging of spindle components, the characterization of their biochemical activities using single-molecule techniques, and theory have advanced our knowledge. Taken together, these advances suggest that a full understanding of spindle assembly and function may soon be possible.
Copyright © 2015 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
References
-
- Akhmanova A, Hoogenraad CC, Drabek K, Stepanova T, Dortland B, Verkerk T, Vermeulen W, Burgering BM, De Zeeuw CI, Grosveld F, et al. 2001. Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell 104: 923–935. - PubMed
-
- Al-Bassam J, Larsen NA, Hyman AA, Harrison SC. 2007. Crystal structure of a TOG domain: Conserved features of XMAP215/Dis1-family TOG domains and implications for tubulin binding. Structure 15: 355–362. - PubMed
-
- Ananthanarayanan V, Schattat M, Vogel SK, Krull A, Pavin N, Tolić-Nørrelykke IM. 2013. Dynein motion switches from diffusive to directed upon cortical anchoring. Cell 153: 1526–1536. - PubMed
-
- Asenjo AB, Chatterjee C, Tan D, DePaoli V, Rice WJ, Diaz-Avalos R, Silvestry M, Sosa H. 2013. Structural model for tubulin recognition and deformation by kinesin-13 microtubule depolymerases. Cell Rep 3: 759–768. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
