Structural Components of Synaptic Plasticity and Memory Consolidation
- PMID: 26134321
- PMCID: PMC4484970
- DOI: 10.1101/cshperspect.a021758
Structural Components of Synaptic Plasticity and Memory Consolidation
Abstract
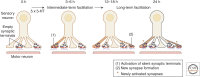
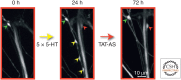
Consolidation of implicit memory in the invertebrate Aplysia and explicit memory in the mammalian hippocampus are associated with remodeling and growth of preexisting synapses and the formation of new synapses. Here, we compare and contrast structural components of the synaptic plasticity that underlies these two distinct forms of memory. In both cases, the structural changes involve time-dependent processes. Thus, some modifications are transient and may contribute to early formative stages of long-term memory, whereas others are more stable, longer lasting, and likely to confer persistence to memory storage. In addition, we explore the possibility that trans-synaptic signaling mechanisms governing de novo synapse formation during development can be reused in the adult for the purposes of structural synaptic plasticity and memory storage. Finally, we discuss how these mechanisms set in motion structural rearrangements that prepare a synapse to strengthen the same memory and, perhaps, to allow it to take part in other memories as a basis for understanding how their anatomical representation results in the enhanced expression and storage of memories in the brain.
Copyright © 2015 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures







References
-
- Abraham WC, Bear MF. 1996. Metaplasticity: The plasticity of synaptic plasticity. Trends Neurosci 19: 126–130. - PubMed
-
- Abraham WC, Huggett A. 1997. Induction and reversal of long-term potentiation by repeated high-frequency stimulation in rat hippocampal slices. Hippocampus 7: 137–145. - PubMed
-
- Abraham WC, Tate WP. 1997. Metaplasticity: A new vista across the field of synaptic plasticity. Prog Neurobiol 52: 303–323. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
