Nano-sized Superlattice Clusters Created by Oxygen Ordering in Mechanically Alloyed Fe Alloys
- PMID: 26134420
- PMCID: PMC4650680
- DOI: 10.1038/srep11772
Nano-sized Superlattice Clusters Created by Oxygen Ordering in Mechanically Alloyed Fe Alloys
Abstract
Creating and maintaining precipitates coherent with the host matrix, under service conditions is one of the most effective approaches for successful development of alloys for high temperature applications; prominent examples include Ni- and Co-based superalloys and Al alloys. While ferritic alloys are among the most important structural engineering alloys in our society, no reliable coherent precipitates stable at high temperatures have been found for these alloys. Here we report discovery of a new, nano-sized superlattice (NSS) phase in ball-milled Fe alloys, which maintains coherency with the BCC matrix up to at least 913 °C. Different from other precipitates in ferritic alloys, this NSS phase is created by oxygen-ordering in the BCC Fe matrix. It is proposed that this phase has a chemistry of Fe3O and a D03 crystal structure and becomes more stable with the addition of Zr. These nano-sized coherent precipitates effectively double the strength of the BCC matrix above that provided by grain size reduction alone. This discovery provides a new opportunity for developing high-strength ferritic alloys for high temperature applications.
Figures


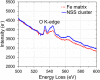
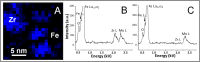
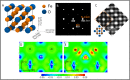
 m. The inset shows orientation relationship between Fe3O unit cell and α-Fe unit cell, i.e. α-Fe [100]// Fe3O [100], α-Fe [010]// Fe3O [010], α-Fe [001]// Fe3O [001]. (B) Simulated [001] diffraction pattern of the proposed Fe3O structure (here Fe3O {020} reflections correspond to BCC {010} superlattice reflections). (C) Experimentally observed [001] HRTEM image (unalloyed Fe sample) compared with the simulated HRTEM image of [001] projection of the proposed Fe3O structure (inset within an orange dotted box), showing good agreement. The [001] structure projection of Fe3O is shown in corner (left bottom). (D) Differential charge density map of the Fe3O unit cell in (1
m. The inset shows orientation relationship between Fe3O unit cell and α-Fe unit cell, i.e. α-Fe [100]// Fe3O [100], α-Fe [010]// Fe3O [010], α-Fe [001]// Fe3O [001]. (B) Simulated [001] diffraction pattern of the proposed Fe3O structure (here Fe3O {020} reflections correspond to BCC {010} superlattice reflections). (C) Experimentally observed [001] HRTEM image (unalloyed Fe sample) compared with the simulated HRTEM image of [001] projection of the proposed Fe3O structure (inset within an orange dotted box), showing good agreement. The [001] structure projection of Fe3O is shown in corner (left bottom). (D) Differential charge density map of the Fe3O unit cell in (1 0) plane without Zr. (E) Differential charge density map of Fe3O unit cell in (1
0) plane without Zr. (E) Differential charge density map of Fe3O unit cell in (1 0) plane with one Fe atom replaced by Zr. The unit of charge density is e/Å3.
0) plane with one Fe atom replaced by Zr. The unit of charge density is e/Å3.References
-
- Viswanathan R., Coleman K. & Rao U. Materials for ultra-supercritical coal-fired power plant boilers. Int. J. Pres. Ves. Pip. 83, 778–783 (2006).
-
- Viswanathan R. et al. US program on materials technology for ultra-supercritical coal power plants. J. Mater. Eng. Perform. 14, 281–292 (2005).
-
- Argon A. S. Strengthening mechanisms in crystal plasticity. (Oxford University Press: Oxford, , 2008).
-
- Edwards G. A., Stiller K., Dunlop G. L. & Couper M. J. The precipitation sequence in Al-Mg-Si alloys. Acta Mater. 46, 3893–3904 (1998).
-
- Reed R. C. The superalloys: fundamentals and applications. (Cambridge University Press, 2006).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

