The emergence of extracellular vesicles in urology: fertility, cancer, biomarkers and targeted pharmacotherapy
- PMID: 26134460
- PMCID: PMC4488336
- DOI: 10.3402/jev.v4.23815
The emergence of extracellular vesicles in urology: fertility, cancer, biomarkers and targeted pharmacotherapy
Abstract
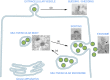
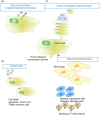
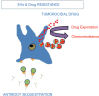
Extracellular vesicles (EV) are small membrane-bound vesicles enriched in a selective repertoire of mRNA, miRNA, proteins and cell surface receptors from parental cells and are actively involved in the transmission of inter and intracellular signals. Cancer cells produce EV that contain cargo including DNA, mRNA, miRNA and proteins that allow EV to create epigenetic changes in target cells both locally and systemically. Cancer-derived EV play critical roles in tumorigenesis, cancer cell migration, metastasis, evasion of host immune defense, chemoresistance, and they promote a premetastatic niche favourable to micrometastatic seeding. Their unique molecular profiles acquired from originator cells and their presence in numerous body fluids, including blood and urine, make them promising candidates as biomarkers for prostate, renal and bladder cancers. EV may ultimately serve as targets for therapy and as platforms for personalized medicine in urology. As urologic malignancy comprises 28% of new solid tumour diagnoses and 15% of cancer-related deaths, EV-related research is rapidly emerging and providing unique insights into disease progression. In this report, we review the current literature on EV in the setting of genitourinary fertility and malignancy.
Keywords: biomarkers; extracellular vesicles; genitourinary fertility and malignancy; prostasomes; prostate, renal and bladder cancer.
Figures

Similar articles
-
Extracellular Vesicles and Their Role in Urologic Malignancies.Eur Urol. 2016 Aug;70(2):323-31. doi: 10.1016/j.eururo.2016.02.046. Epub 2016 Feb 28. Eur Urol. 2016. PMID: 26924769 Review.
-
A Systematic Review of Extracellular Vesicle-Derived Piwi-Interacting RNA in Human Body Fluid and Its Role in Disease Progression.Tissue Eng Part C Methods. 2022 Oct;28(10):511-528. doi: 10.1089/ten.TEC.2022.0092. Tissue Eng Part C Methods. 2022. PMID: 35959742
-
Extracellular Vesicles: Recent Developments in Technology and Perspectives for Cancer Liquid Biopsy.Recent Results Cancer Res. 2020;215:319-344. doi: 10.1007/978-3-030-26439-0_17. Recent Results Cancer Res. 2020. PMID: 31605237 Review.
-
Defining candidate mRNA and protein EV biomarkers to discriminate ccRCC and pRCC from non-malignant renal cells in vitro.Med Oncol. 2021 Jul 31;38(9):105. doi: 10.1007/s12032-021-01554-2. Med Oncol. 2021. PMID: 34331598 Free PMC article.
-
Application of extracellular vesicles in the diagnosis and treatment of prostate cancer: Implications for clinical practice.Crit Rev Oncol Hematol. 2021 Nov;167:103495. doi: 10.1016/j.critrevonc.2021.103495. Epub 2021 Oct 13. Crit Rev Oncol Hematol. 2021. PMID: 34655743 Review.
Cited by
-
Metabolic characteristics of large and small extracellular vesicles from pleural effusion reveal biomarker candidates for the diagnosis of tuberculosis and malignancy.J Extracell Vesicles. 2020 Jul 14;9(1):1790158. doi: 10.1080/20013078.2020.1790158. J Extracell Vesicles. 2020. PMID: 32944177 Free PMC article.
-
Human Transmembrane Serine Protease 2 (TMPRSS2) on Human Seminal Fluid Extracellular Vesicles Is Proteolytically Active.J Extracell Vesicles. 2025 Mar;14(3):e70061. doi: 10.1002/jev2.70061. J Extracell Vesicles. 2025. PMID: 40091430 Free PMC article.
-
Exosomes in Breast Cancer - Mechanisms of Action and Clinical Potential.Mol Cancer Res. 2021 Jun;19(6):935-945. doi: 10.1158/1541-7786.MCR-20-0952. Epub 2021 Feb 24. Mol Cancer Res. 2021. PMID: 33627501 Free PMC article. Review.
-
Secreted MicroRNA to Predict Embryo Implantation Outcome: From Research to Clinical Diagnostic Application.Front Cell Dev Biol. 2020 Sep 22;8:586510. doi: 10.3389/fcell.2020.586510. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33072767 Free PMC article. Review.
-
Biocompatible Nanomaterials as an Emerging Technology in Reproductive Health; a Focus on the Male.Front Physiol. 2021 Nov 11;12:753686. doi: 10.3389/fphys.2021.753686. eCollection 2021. Front Physiol. 2021. PMID: 34858208 Free PMC article. Review.
References
-
- Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. - PubMed
-
- Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3 26913, doi: http://dx.doi.org/10.3402/jev.v3.26913. - DOI - PMC - PubMed
-
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. - PubMed
-
- El Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–57. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

