Engineered and Native Coenzyme B12-dependent Isovaleryl-CoA/Pivalyl-CoA Mutase
- PMID: 26134562
- PMCID: PMC4536452
- DOI: 10.1074/jbc.M115.646299
Engineered and Native Coenzyme B12-dependent Isovaleryl-CoA/Pivalyl-CoA Mutase
Abstract
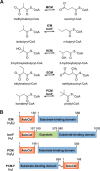
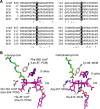
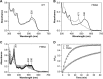
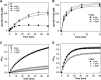
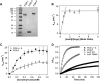
Adenosylcobalamin-dependent isomerases catalyze carbon skeleton rearrangements using radical chemistry. We have recently demonstrated that an isobutyryl-CoA mutase variant, IcmF, a member of this enzyme family that catalyzes the interconversion of isobutyryl-CoA and n-butyryl-CoA also catalyzes the interconversion between isovaleryl-CoA and pivalyl-CoA, albeit with low efficiency and high susceptibility to inactivation. Given the biotechnological potential of the isovaleryl-CoA/pivalyl-CoA mutase (PCM) reaction, we initially attempted to engineer IcmF to be a more proficient PCM by targeting two active site residues predicted based on sequence alignments and crystal structures, to be key to substrate selectivity. Of the eight mutants tested, the F598A mutation was the most robust, resulting in an ∼17-fold increase in the catalytic efficiency of the PCM activity and a concomitant ∼240-fold decrease in the isobutyryl-CoA mutase activity compared with wild-type IcmF. Hence, mutation of a single residue in IcmF tuned substrate specificity yielding an ∼4000-fold increase in the specificity for an unnatural substrate. However, the F598A mutant was even more susceptible to inactivation than wild-type IcmF. To circumvent this limitation, we used bioinformatics analysis to identify an authentic PCM in genomic databases. Cloning and expression of the putative AdoCbl-dependent PCM with an α2β2 heterotetrameric organization similar to that of isobutyryl-CoA mutase and a recently characterized archaeal methylmalonyl-CoA mutase, allowed demonstration of its robust PCM activity. To simplify kinetic analysis and handling, a variant PCM-F was generated in which the αβ subunits were fused into a single polypeptide via a short 11-amino acid linker. The fusion protein, PCM-F, retained high PCM activity and like PCM, was resistant to inactivation. Neither PCM nor PCM-F displayed detectable isobutyryl-CoA mutase activity, demonstrating that PCM represents a novel 5'-deoxyadenosylcobalamin-dependent acyl-CoA mutase. The newly discovered PCM and the derivative PCM-F, have potential applications in bioremediation of pivalic acid found in sludge, in stereospecific synthesis of C5 carboxylic acids and alcohols, and in the production of potential commodity and specialty chemicals.
Keywords: adenosylcobalamin (AdoCbl); cofactor; enzyme; enzyme catalysis; enzyme kinetics; enzyme mutation; isomerase.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Banerjee R. (2003) Radical carbon skeleton rearrangements: catalysis by coenzyme B12-dependent mutases. Chem. Rev. 103, 2083–2094 - PubMed
-
- Banerjee R. (2001) Radical peregrinations catalyzed by coenzyme B12-dependent enzymes. Biochemistry 40, 6191–6198 - PubMed
-
- Frey P. A. (2014) Travels with carbon-centered radicals: 5′-deoxyadenosine and 5′-deoxyadenosine-5′-yl in radical enzymology. Acc. Chem. Res. 47, 540–549 - PubMed
-
- Dowling D. P., Croft A. K., Drennan C. L. (2012) Radical use of Rossmann and TIM barrel architectures for controlling coenzyme B12 chemistry. Annu. Rev. Biophys. 41, 403–427 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

