Metal Fluoride Inhibition of a P-type H+ Pump: STABILIZATION OF THE PHOSPHOENZYME INTERMEDIATE CONTRIBUTES TO POST-TRANSLATIONAL PUMP ACTIVATION
- PMID: 26134563
- PMCID: PMC4536445
- DOI: 10.1074/jbc.M115.639385
Metal Fluoride Inhibition of a P-type H+ Pump: STABILIZATION OF THE PHOSPHOENZYME INTERMEDIATE CONTRIBUTES TO POST-TRANSLATIONAL PUMP ACTIVATION
Abstract
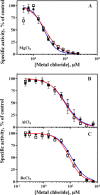
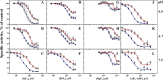
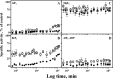
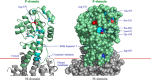
The plasma membrane H(+)-ATPase is a P-type ATPase responsible for establishing electrochemical gradients across the plasma membrane in fungi and plants. This essential proton pump exists in two activity states: an autoinhibited basal state with a low turnover rate and a low H(+)/ATP coupling ratio and an activated state in which ATP hydrolysis is tightly coupled to proton transport. Here we characterize metal fluorides as inhibitors of the fungal enzyme in both states. In contrast to findings for other P-type ATPases, inhibition of the plasma membrane H(+)-ATPase by metal fluorides was partly reversible, and the stability of the inhibition varied with the activation state. Thus, the stability of the ATPase inhibitor complex decreased significantly when the pump transitioned from the activated to the basal state, particularly when using beryllium fluoride, which mimics the bound phosphate in the E2P conformational state. Taken together, our results indicate that the phosphate bond of the phosphoenzyme intermediate of H(+)-ATPases is labile in the basal state, which may provide an explanation for the low H(+)/ATP coupling ratio of these pumps in the basal state.
Keywords: H+-ATPase; plasma membrane; post-translational modification (PTM); proton pump; proton transport.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Goffeau A., Slayman C. W. (1981) The proton-translocating ATPase of the fungal plasma membrane. Biochim. Biophys. Acta 639, 197–223 - PubMed
-
- Lefebvre B., Boutry M., Morsomme P. (2003) The yeast and plant plasma membrane H+ pump ATPase: divergent regulation for the same function. Prog. Nucleic Acid Res. Mol. Biol. 74, 203–237 - PubMed
-
- Morth J. P., Pedersen B. P., Buch-Pedersen M. J., Andersen J. P., Vilsen B., Palmgren M. G., Nissen P. (2011) A structural overview of the plasma membrane Na+,K+-ATPase and H+-ATPase ion pumps. Nat. Rev. Mol. Cell Biol. 12, 60–70 - PubMed
-
- Slayman C. L. (1987) The plasma membrane ATPase of Neurospora: a proton-pumping electroenzyme. J. Bioenerg. Biomembr. 19, 1–20 - PubMed
-
- Palmgren M. G., Nissen P. (2011) P-type ATPases. Annu. Rev. Biophys. 40, 243–266 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

