Structure and Function of the Hypertension Variant A486V of G Protein-coupled Receptor Kinase 4
- PMID: 26134571
- PMCID: PMC4536442
- DOI: 10.1074/jbc.M115.648907
Structure and Function of the Hypertension Variant A486V of G Protein-coupled Receptor Kinase 4
Abstract
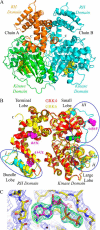
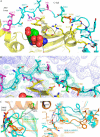
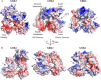
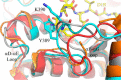
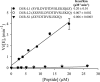
G-protein-coupled receptor (GPCR) kinases (GRKs) bind to and phosphorylate GPCRs, initiating the process of GPCR desensitization and internalization. GRK4 is implicated in the regulation of blood pressure, and three GRK4 polymorphisms (R65L, A142V, and A486V) are associated with hypertension. Here, we describe the 2.6 Å structure of human GRK4α A486V crystallized in the presence of 5'-adenylyl β,γ-imidodiphosphate. The structure of GRK4α is similar to other GRKs, although slight differences exist within the RGS homology (RH) bundle subdomain, substrate-binding site, and kinase C-tail. The RH bundle subdomain and kinase C-terminal lobe form a strikingly acidic surface, whereas the kinase N-terminal lobe and RH terminal subdomain surfaces are much more basic. In this respect, GRK4α is more similar to GRK2 than GRK6. A fully ordered kinase C-tail reveals interactions linking the C-tail with important determinants of kinase activity, including the αB helix, αD helix, and the P-loop. Autophosphorylation of wild-type GRK4α is required for full kinase activity, as indicated by a lag in phosphorylation of a peptide from the dopamine D1 receptor without ATP preincubation. In contrast, this lag is not observed in GRK4α A486V. Phosphopeptide mapping by mass spectrometry indicates an increased rate of autophosphorylation of a number of residues in GRK4α A486V relative to wild-type GRK4α, including Ser-485 in the kinase C-tail.
Keywords: G-protein-coupled receptor (GPCR); GRK4; dopamine D1 receptor; hypertension; kinetics; phosphorylation; serine/threonine protein kinase; x-ray crystallography.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Ribas C., Penela P., Murga C., Salcedo A., García-Hoz C., Jurado-Pueyo M., Aymerich I., Mayor F., Jr. (2007) The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochim. Biophys. Acta 1768, 913–922 - PubMed
-
- Homan K. T., Glukhova A., Tesmer J. J. (2013) Regulation of G protein-coupled receptor kinases by phospholipids. Curr. Med. Chem. 20, 39–46 - PubMed
-
- Pitcher J. A., Fredericks Z. L., Stone W. C., Premont R. T., Stoffel R. H., Koch W. J., Lefkowitz R. J. (1996) Phosphatidylinositol 4,5-bisphosphate (PIP2)-enhanced G protein-coupled receptor kinase (GRK) activity. Location, structure, and regulation of the PIP2 binding site distinguishes the GRK subfamilies. J. Biol. Chem. 271, 24907–24913 - PubMed
-
- Pronin A. N., Satpaev D. K., Slepak V. Z., Benovic J. L. (1997) Regulation of G protein-coupled receptor kinases by calmodulin and localization of the calmodulin binding domain. J. Biol. Chem. 272, 18273–18280 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

