NMDA Receptor Plasticity in the Hypothalamic Paraventricular Nucleus Contributes to the Elevated Blood Pressure Produced by Angiotensin II
- PMID: 26134639
- PMCID: PMC4571498
- DOI: 10.1523/JNEUROSCI.2301-14.2015
NMDA Receptor Plasticity in the Hypothalamic Paraventricular Nucleus Contributes to the Elevated Blood Pressure Produced by Angiotensin II
Abstract
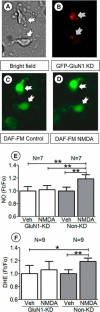
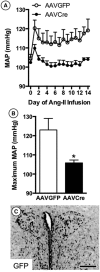
Hypertension induced by angiotensin II (Ang II) is associated with glutamate-dependent dysregulation of the hypothalamic paraventricular nucleus (PVN). Many forms of glutamate-dependent plasticity are mediated by NMDA receptor GluN1 subunit expression and the distribution of functional receptor to the plasma membrane of dendrites. Here, we use a combined ultrastructural and functional analysis to examine the relationship between PVN NMDA receptors and the blood pressure increase induced by chronic infusion of a low dose of Ang II. We report that the increase in blood pressure produced by a 2 week administration of a subpressor dose of Ang II results in an elevation in plasma membrane GluN1 in dendrites of PVN neurons in adult male mice. The functional implications of these observations are further demonstrated by the finding that GluN1 deletion in PVN neurons attenuated the Ang II-induced increases in blood pressure. These results indicate that NMDA receptor plasticity in PVN neurons significantly contributes to the elevated blood pressure mediated by Ang II.
Keywords: gene deletion; gene therapy; hypertension; hypothalamus; synaptic plasticity.
Copyright © 2015 the authors 0270-6474/15/359558-10$15.00/0.
Figures



Similar articles
-
Redistribution of NMDA Receptors in Estrogen-Receptor-β-Containing Paraventricular Hypothalamic Neurons following Slow-Pressor Angiotensin II Hypertension in Female Mice with Accelerated Ovarian Failure.Neuroendocrinology. 2017;104(3):239-256. doi: 10.1159/000446073. Epub 2016 Apr 15. Neuroendocrinology. 2017. PMID: 27078860 Free PMC article.
-
Sex differences in NMDA GluN1 plasticity in rostral ventrolateral medulla neurons containing corticotropin-releasing factor type 1 receptor following slow-pressor angiotensin II hypertension.Neuroscience. 2015 Oct 29;307:83-97. doi: 10.1016/j.neuroscience.2015.08.029. Epub 2015 Aug 22. Neuroscience. 2015. PMID: 26306872 Free PMC article.
-
Angiotensin II slow-pressor hypertension enhances NMDA currents and NOX2-dependent superoxide production in hypothalamic paraventricular neurons.Am J Physiol Regul Integr Comp Physiol. 2013 Jun 15;304(12):R1096-106. doi: 10.1152/ajpregu.00367.2012. Epub 2013 Apr 10. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 23576605 Free PMC article.
-
Slow-pressor angiotensin II hypertension and concomitant dendritic NMDA receptor trafficking in estrogen receptor β-containing neurons of the mouse hypothalamic paraventricular nucleus are sex and age dependent.J Comp Neurol. 2014 Sep 1;522(13):3075-90. doi: 10.1002/cne.23569. J Comp Neurol. 2014. PMID: 24639345 Free PMC article.
-
Angiotensin-II-induced reactive oxygen species along the SFO-PVN-RVLM pathway: implications in neurogenic hypertension.Braz J Med Biol Res. 2011 Sep;44(9):871-6. doi: 10.1590/s0100-879x2011007500088. Epub 2011 Jul 8. Braz J Med Biol Res. 2011. PMID: 21755262 Review.
Cited by
-
Ion Channels in the Paraventricular Hypothalamic Nucleus (PVN); Emerging Diversity and Functional Roles.Front Physiol. 2018 Jul 6;9:760. doi: 10.3389/fphys.2018.00760. eCollection 2018. Front Physiol. 2018. PMID: 30034342 Free PMC article. Review.
-
α2δ-1-Dependent NMDA Receptor Activity in the Hypothalamus Is an Effector of Genetic-Environment Interactions That Drive Persistent Hypertension.J Neurosci. 2021 Jul 28;41(30):6551-6563. doi: 10.1523/JNEUROSCI.0346-21.2021. Epub 2021 Jun 30. J Neurosci. 2021. PMID: 34193557 Free PMC article.
-
Central Ang II (Angiotensin II)-Mediated Sympathoexcitation: Role for HIF-1α (Hypoxia-Inducible Factor-1α) Facilitated Glutamatergic Tone in the Paraventricular Nucleus of the Hypothalamus.Hypertension. 2021 Jan;77(1):147-157. doi: 10.1161/HYPERTENSIONAHA.120.16002. Epub 2020 Dec 8. Hypertension. 2021. PMID: 33296248 Free PMC article.
-
Persistence of post-stress blood pressure elevation requires activation of astrocytes.Sci Rep. 2024 Oct 3;14(1):22984. doi: 10.1038/s41598-024-73345-4. Sci Rep. 2024. PMID: 39363030 Free PMC article.
-
Sex Differences in the Rat Hippocampal Opioid System After Oxycodone Conditioned Place Preference.Neuroscience. 2018 Nov 21;393:236-257. doi: 10.1016/j.neuroscience.2018.10.002. Epub 2018 Oct 11. Neuroscience. 2018. PMID: 30316908 Free PMC article.
References
-
- Beckerman MA, Glass MJ. The NMDA-NR1 receptor subunit and the mu-opioid receptor are expressed in somatodendritic compartments of central nucleus of the amygdala neurons projecting to the bed nucleus of the stria terminalis. Exp Neurol. 2012;234:112–126. doi: 10.1016/j.expneurol.2011.12.034. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL09657/HL/NHLBI NIH HHS/United States
- MH40342/MH/NIMH NIH HHS/United States
- R01 DA004600/DA/NIDA NIH HHS/United States
- R01 MH040342/MH/NIMH NIH HHS/United States
- NS89323/NS/NINDS NIH HHS/United States
- HL063887/HL/NHLBI NIH HHS/United States
- R01 HL063887/HL/NHLBI NIH HHS/United States
- R01 HL098351/HL/NHLBI NIH HHS/United States
- P01 HL084207/HL/NHLBI NIH HHS/United States
- DA04600/DA/NIDA NIH HHS/United States
- DA024030/DA/NIDA NIH HHS/United States
- R37 MH040342/MH/NIMH NIH HHS/United States
- F32 HL009657/HL/NHLBI NIH HHS/United States
- HL098351/HL/NHLBI NIH HHS/United States
- R37 NS089323/NS/NINDS NIH HHS/United States
- P01 HL096571/HL/NHLBI NIH HHS/United States
- R01 DA024030/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
