Differential Effect of Neuropeptides on Excitatory Synaptic Transmission in Human Epileptic Hippocampus
- PMID: 26134645
- PMCID: PMC6605141
- DOI: 10.1523/JNEUROSCI.3973-14.2015
Differential Effect of Neuropeptides on Excitatory Synaptic Transmission in Human Epileptic Hippocampus
Abstract
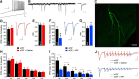
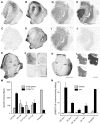
Development of novel disease-modifying treatment strategies for neurological disorders, which at present have no cure, represents a major challenge for today's neurology. Translation of findings from animal models to humans represents an unresolved gap in most of the preclinical studies. Gene therapy is an evolving innovative approach that may prove useful for clinical applications. In animal models of temporal lobe epilepsy (TLE), gene therapy treatments based on viral vectors encoding NPY or galanin have been shown to effectively suppress seizures. However, how this translates to human TLE remains unknown. A unique possibility to validate these animal studies is provided by a surgical therapeutic approach, whereby resected epileptic tissue from temporal lobes of pharmacoresistant patients are available for neurophysiological studies in vitro. To test whether NPY and galanin have antiepileptic actions in human epileptic tissue as well, we applied these neuropeptides directly to human hippocampal slices in vitro. NPY strongly decreased stimulation-induced EPSPs in dentate gyrus and CA1 (up to 30 and 55%, respectively) via Y2 receptors, while galanin had no significant effect. Receptor autoradiographic binding revealed the presence of both NPY and galanin receptors, while functional receptor binding was only detected for NPY, suggesting that galanin receptor signaling may be impaired. These results underline the importance of validating findings from animal studies in human brain tissue, and advocate for NPY as a more appropriate candidate than galanin for future gene therapy trials in pharmacoresistant TLE patients.
Keywords: NPY; galanin; gene therapy; hippocampus; temporal lobe epilepsy.
Copyright © 2015 the authors 0270-6474/15/359622-10$15.00/0.
Figures





Similar articles
-
Y5 receptors mediate neuropeptide Y actions at excitatory synapses in area CA3 of the mouse hippocampus.J Neurophysiol. 2002 Jan;87(1):558-66. doi: 10.1152/jn.00532.2001. J Neurophysiol. 2002. PMID: 11784771
-
Blockade of neuropeptide Y(2) receptors and suppression of NPY's anti-epileptic actions in the rat hippocampal slice by BIIE0246.Br J Pharmacol. 2002 Jun;136(4):502-9. doi: 10.1038/sj.bjp.0704751. Br J Pharmacol. 2002. PMID: 12055128 Free PMC article.
-
Neuropeptide Y(5) receptors reduce synaptic excitation in proximal subiculum, but not epileptiform activity in rat hippocampal slices.J Neurophysiol. 2000 Feb;83(2):723-34. doi: 10.1152/jn.2000.83.2.723. J Neurophysiol. 2000. PMID: 10669488
-
Overexpression of NPY and Y2 receptors in epileptic brain tissue: an endogenous neuroprotective mechanism in temporal lobe epilepsy?Neuropeptides. 2004 Aug;38(4):245-52. doi: 10.1016/j.npep.2004.05.004. Neuropeptides. 2004. PMID: 15337376 Review.
-
Gene therapy in epilepsy: the focus on NPY.Peptides. 2007 Feb;28(2):377-83. doi: 10.1016/j.peptides.2006.07.025. Epub 2006 Dec 28. Peptides. 2007. PMID: 17196301 Review.
Cited by
-
A Matter of Genes: The Hurdles of Gene Therapy for Epilepsy.Epilepsy Curr. 2019 Jan;19(1):38-43. doi: 10.1177/1535759718822846. Epub 2019 Feb 12. Epilepsy Curr. 2019. PMID: 30838918 Free PMC article.
-
Glioblastoma CD105+ cells define a SOX2- cancer stem cell-like subpopulation in the pre-invasive niche.Acta Neuropathol Commun. 2022 Aug 29;10(1):126. doi: 10.1186/s40478-022-01422-8. Acta Neuropathol Commun. 2022. PMID: 36038950 Free PMC article.
-
A robust ex vivo experimental platform for molecular-genetic dissection of adult human neocortical cell types and circuits.Sci Rep. 2018 May 30;8(1):8407. doi: 10.1038/s41598-018-26803-9. Sci Rep. 2018. PMID: 29849137 Free PMC article.
-
Dynorphin-based "release on demand" gene therapy for drug-resistant temporal lobe epilepsy.EMBO Mol Med. 2019 Oct;11(10):e9963. doi: 10.15252/emmm.201809963. Epub 2019 Sep 5. EMBO Mol Med. 2019. PMID: 31486590 Free PMC article.
-
Seizure-induced overexpression of NPY induces epileptic tolerance in a mouse model of spontaneous recurrent seizures.Front Mol Neurosci. 2022 Oct 13;15:974784. doi: 10.3389/fnmol.2022.974784. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36311021 Free PMC article.
References
-
- Agasse F, Xapelli S, Coronas V, Christiansen SH, Rosa AI, Sardá-Arroyo L, Santos T, Ferreira R, Schitine C, Harnois T, Bourmeyster N, Bragança J, Bernardino L, Malva JO, Woldbye DP. Galanin promotes neuronal differentiation in murine subventricular zone cell cultures. Stem Cells Dev. 2013;22:1693–1708. doi: 10.1089/scd.2012.0161. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
